FDA Approves Bayer's Newest Birth Control Device
By Jof Enriquez,
Follow me on Twitter @jofenriq

Bayer announced that the U.S. Food and Drug Administration (FDA) has approved the company's newest intrauterine device (IUD), Kyleena (levonorgestrel-releasing intrauterine system) 19.5 mg, for the reversible, long-acting prevention of pregnancy for up to five years.
An IUD is a small, T-shaped device that’s placed non-surgically in the uterus by a healthcare provider during a routine in-office visit. Bayer already markets the Skyla and Mirena brand of IUDs which, like Kyleena, release the progestin hormone levonorgestrel. The hormone works by preventing the release of an egg from the ovary or preventing fertilization of the egg by sperm (male reproductive cells), and also by changing the lining of the uterus (womb) to prevent pregnancy.
"Data show that the use of effective, long-acting birth control methods including intrauterine devices – or IUDs – have helped to reduce unintended pregnancies in the United States but we still have a long way to go," said Anita L. Nelson, M.D., Professor and Chair, Obstetrics and Gynecology at Western University of Health Sciences, Pomona, Calif, in a news release.
Bayer says that Kyleena will be available by prescription only in October 2016.
A clinical trial of 1,452 women aged 18-35 in 11 countries in Europe, Latin America, the U.S. and Canada, showed the contraceptive efficacy of Kyleena, as evidenced by favorable Pearl Index (PI) rates, said Bayer.
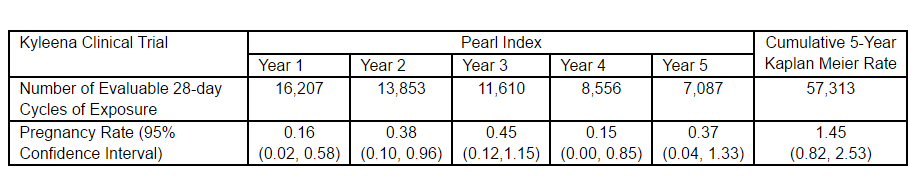
Demonstrating reversibility were the 71 percent of 163 women who desired pregnancy after study discontinuation who successfully conceived within 12 months after removal of the device. Among the study participants, the most common adverse reactions reported were vulvovaginitis (24 percent), ovarian cyst (22 percent), abdominal pain/pelvic pain (21 percent), headache/migraine (15 percent), and acne/seborrhea (15 percent).
Bayer claims that the use of long-acting reversible contraception (LARCs), which includes IUDs and implants like Kyleena, Skyla, and Mirena, has increased nearly five-fold in the last decade. LARCs are more effective than other contraceptives, such as pills and patches, and are nearly as effective as sterilization, according to the U.S. Centers for Disease Control and Prevention, reports NewsMax.
FDA in February 2015 also approved another levonorgestrel-releasing IUD, Liletta, manufactured by Actavis (now Allergan). The contraceptive device is effective for up to three years.
