How To Develop A Quality Culture In Your Medical Device Manufacturing Operation

By Gerry Creaner, President, GetReskilled
The lengths to which medical device companies go to deliver a quality product vary widely, but clearly there are some commonalities with regard to the development and maintenance of a quality management system that produces consistently excellent products. One of these has to do with the amount of attention given to manufacturing operator and technician training.
Thinking Ahead
With the FDA Safety and Innovation Act (FDASIA) providing funding for more audits, and the FDA’s current focus on measuring, incentivizing, and rewarding the development of a quality culture across all levels of a manufacturing organization, the industry is currently experiencing an uptick in scrutiny.
What can be done now to stem the inevitable increase in noted deficiencies and 483s? Purposeful disregard for current good manufacturing practice (cGMP) guidelines and standard operating procedures (SOPs) is rare, but there are other nagging situations, such as operator error, where quality can be anticipated and improved. One company has decided to attack this head-on and give companies the tools to anticipate quality issues within their workforce and address them proactively.
GetReSkilled uses video learning and a tool called the Behavioral Positioning System to anticipate and coach quality capability learning among the operator and technician workforce.
Rote Behavior Syndrome
Often quality issues that are traced back to operator error are tied to the tedium of job performance, as operators perform the same task repeatedly. Rote behavior can haunt a cleanroom suite, or manufacturing floor. Although the individuals often know the SOPs quite well and are intimately familiar with GMPs, mistakes can be, and are, made — albeit inadvertently. This is because workers may not have a true understanding of the “why” behind those guidelines.
It should come as no surprise that culture can also play a role in quality problem reporting. If bringing potential quality problems to the attention of superiors is not acceptable in certain cultures (because it is not acceptable to question authority), this means that even the most technically perfect set of SOPs and cGMP training sessions will not help.
To ensure that your company has an audit-ready quality program from the “ground floor up,” you can and should give operators more detail on the “whys” of cGMP, including more than just the usual list of SOP instructions and step-by-steps that is the norm in most operator training.
Science Trumps Culture
Addressing the science behind medical device manufacturing and packaging is critical to allow operators to understand why a certain guideline is written a certain way and why it is important. A smart quality management program will not ignore the cultural realities of manufacturing in China, or other Eastern countries, where questioning a superior might be considered a sign of disrespect. Smart quality managers now recognize that using science-based training can help operators to ask questions about the “science,” rather than be in the position of questioning superiors about a quality issue.
Make It Personal
Connecting the process and device to actual disease symptoms and specifics, along with clearly identifying how operators’ medical device output is curing or easing those symptoms, has also proven to be quite effective (i.e., making their job “personal”). Connecting operators to patients and the personal struggle of real families that use such devices completely changes the connectivity and adherence to the quality guidelines.
Measurement Makes Perfect Sense
Once you have embarked on this type of capability building within the organization, either in a classroom setting or via video snippets delivered prior to each shift, don’t wait until the FDA shows up to determine whether or not your company has been successful.
For example, a world-leading manufacturer of orthopedic medical technology wanted a more customized approach to training their manufacturing operators. When they saw the customizable, in-depth medical device modules that were possible with GetReskilled’s Behavioral Positioning System, they conducted 12 months of ongoing training for their operations and manufacturing teams across multiple locations. The operators who participated were tested using customized and site-relevant, end-of-session testing, allowing management to measure the increased levels of capability. This device manufacturer also uses the training records in their annual performance reviews.
In an industry of scientists and engineers who are drawn to an industry that measures results, how do you measure improvement on the more subjective side of the business — the quality culture — after capability training is implemented?
Psychometric Testing Can Aid Measurement
A revolutionary new psychometric profiling tool has been developed to identify key characteristics associated with a more highly quality-conscious operator. This concept is innovative and ground-breaking in the arena of life sciences, although other life-critical industries already use psychometric tools on a regular basis, helping to identify manufacturing operators who would be quality-conscious workers by nature.
Both the airline industry and the sterile packaging industry use the psychometric profiling of worker behavior traits to identify those best-suited to their positions. A study conducted at a food-processing organization found that greater worker trait conscientiousness predicted better self-reported food safety behavior. For example, identifying operators who exhibit conscientiousness has been found to relate to being alert to possible contamination issues. Conscientiousness is also associated with operators who would more easily deliver a systematic performance of actions to ensure that food is and stays clean.
How Does It Work?
Psychometric testing first identifies the scenarios that are most predictive of success (i.e., adherence to written guidelines and being verbally comfortable in pointing out quality mistakes to superiors).
Testing is conducted with different scenario-based questions to identify key traits in an operator that indicate how they might behave under those circumstances.
A recent study was conducted in August 2014 of 300 experienced workers who transitioned into operator/technician roles across 50 medical device, pharmaceutical, or biotechnology manufacturing plants. The study, co-sponsored by GetReSkilled and cut-E (a world leader in the design and implementation of innovative online tests and questionnaires for recruitment, selection, and development), sought to reveal key characteristics similar to those used so successfully to identify great quality behavior in other life-critical industries.
The two graphs below show the results of this study in two different ways.
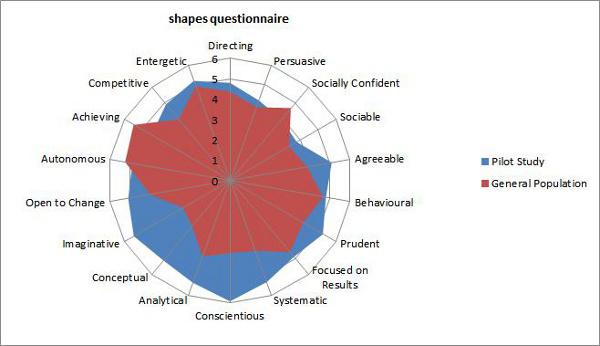
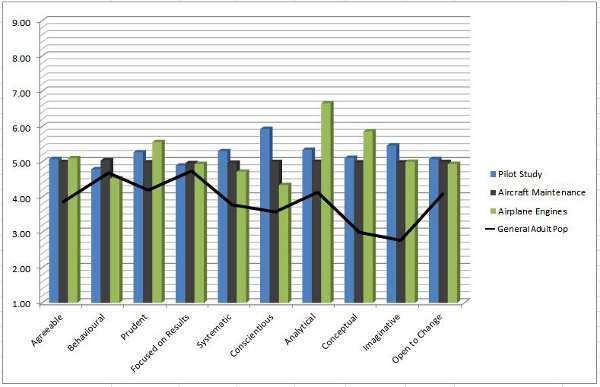
There are clear differences between characteristics exhibited by the general population as opposed to other life-critical industries, such as airplane engine manufacturers. The study demonstrated the high correlation and similarity between the characteristics of medical device operator behavior and the behaviors of other life-critical industry operators.
Clearly, there are characteristics (analytical, imaginative, conceptual) that both life science operators and life-critical airline engine manufacturing operators exhibit at much higher levels. These characteristics can be tested for prior to hire as well. Finding out that your operator workforce may be lacking certain characteristics can reveal a path to build and improve the operator workforce quality mindset.
The real point here is that this type of profile, once developed for your particular organization, can be a benchmark set of characteristics for your medical device organization. The Behavioral Positioning System can then be employed, as described earlier, to train the manufacturing workforce and show improvement in and against their natural quality behavior profile. It can be measured.
Who Will Speak Up?
How do you ensure that a new manufacturing operator at your plant will value adherence to the manufacturing procedures over external direction to do otherwise, say from his superior?
In life-critical industries such as aircraft manufacturing, food safety, or sterile packaging industries, companies have identified traits that reliably predict that individuals with the following scores will adhere to manufacturing procedures over negative external direction from superiors:
- Conscientious (higher score) – pays attention to quality issues; detail-oriented; follows procedures, rules, and regulations
- Planful (higher score) – systematic, organized, and plans ahead
- Imaginative (lower score) – more likely to be distracted due to inattentiveness
- Expressive (lower score) – more likely to get distracted by talking to others
- Change-oriented (lower score) – more likely to be looking and trying out new (unapproved) ways of accomplishing tasks
Employee Appreciation: A Natural Side Effect
Taking the time to offer regular, consistent quality training is an excellent morale boost for employees as well. Individuals appreciate the specificity of the training. Noel O’Brien, a manufacturing team member with Stryker in Cork, Ireland, was an experienced manufacturing worker who came into the GetReskilled curriculum with the hope that he could use the capability training to learn more about the medical device manufacturing field.
In regards to the training, Noel said: “The quality of the course content was excellent, substantial, and covered all aspects associated within the pharmaceutical, biopharmaceutical and medical device industries.”
Another experienced worker in medical device manufacturing, David Masterson, was able to take advantage of the GetReskilled curriculum to learn more about the quality aspects of his operator position, and eventually secure a different job as a quality team member in medical devices with BD (Becton, Dickinson and Company) at its Dublin, Ireland site. Masterson said: “The courses are detailed, easily accessible, great to add to your personal CV and compliment other qualifications.”
What to Do Next
Medical device companies can develop a personalized profile for their individual manufacturing situation, and focus on key characteristics that connect to the highest quality performance by an operator. There will be several results of this effort — profiles of different individuals who would be best-suited to the operation tasks that produce the highest quality products and current staff analysis. Current staff can be tested easily and quickly to determine the type of capability learning that would be best-suited for improving quality reaction and behavior inside an operator population. Then, the supervisor reviews can be re-administered at regular intervals to measure progress.
Video or classroom learning can be used to deliver the “whys” of cGMP, the science, and the personal touch of family-disease connection. Using video learning has been proven to make education “stick,” and can be delivered prior to operator shifts in a shop floor environment to move operators along a behavior improvement curve more efficiently than in a classroom setting.
It makes sense to anticipate that clients and regulatory agencies will all want to see proactive programs in place that go beyond the basic SOP operator training. Instituting Behavioral Positioning System training prior to any audit that might mandate such a change will show a proactive attitude in the event of a future problem. The industry can and should consider this as a tool to demonstrate a best-in-class quality mindset, and, to mirror the words used by Dr. Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research (CDER), during her plenary speech at the 2013 ISPE Annual Meeting: “Develop a quality culture from the ground floor on up, all the way to the boardroom.”
About The Author
Gerry Creaner has over 30 years of experience in the life sciences manufacturing industry across a range of technical, managerial, and business roles. A chemical engineer by profession, he established a very successful engineering consultancy prior to founding GetReskilled, Inc., an online education and learning business focused on the manufacture of safe and effective medicines and medical devices for the public, with particular emphasis on developing these competencies within the operator and technician groups across the global supply chain.
About The Companies
GetReskilled has trained over 3,000 pharmaceutical and medical device operators since its inception, transitioning into video operator training in 2010. Topics are very specific to medical device (and other pharmaceutical and biotech subject matter), and focus on either the science or the personal connection.
cut-e conducts psychometric testing for 14,000,000 individuals annually and is a recognized leader in the industry.
Behavioral Positioning System is a partnership service offered by GetReskilled and cut-e to the medical device, pharmaceutical, and biotechnology industry. The joint product was launched this year at ISPE’s Annual Meeting in Las Vegas, Nevada.
