J&J, Materialise Partner On 3D-Printed Custom Cranio-Facial Implants

By Jof Enriquez,
Follow me on Twitter @jofenriq
Johnson & Johnson's DePuy Synthes is expanding its collaboration with Belgian 3D printing company Materialise to offer patient-specific titanium craniomaxillofacial (CMF) implants under the DePuy Synthes TRUMATCH portfolio.
J&J considers 3D printing as a disruptive technology that will help it deliver personalized healthcare solutions. The company's 3D Printing & Netshape Technologies Center has partnered with over 50 external technology, academic, and government entities on various 3D applications and products.
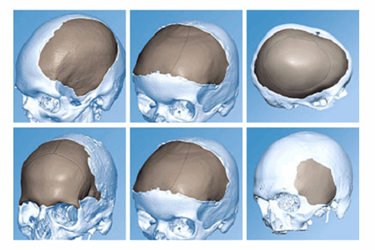
J&J has been working with Materialise since 2010 in the field of personalized solutions for craniofacial surgery. Now, the partnership will expand to offer a personalized, total solution for orthognathic surgery and other craniofacial indications that seamlessly integrates virtual surgical planning, intraoperative patient specific tools, and personalized 3D-printed implants to help surgeons achieve their goals for accuracy and patient outcomes.
The TRUMATCH CMF solution currently includes ProPlan CMF, a Materialise and DePuy Synthes solution for planning and patient-specific guides for CMF surgery, according to TCT Magazine. These custom titanium CMF implants will be available in Australia and throughout Europe, the Middle East, and Africa (EMEA), with the exception of France.
Elmar Zurbriggen, VP, DePuy Synthes in Europe, the Middle East and Africa, stated, "The TRUMATCH CMF Solutions portfolio includes several advanced technologies for facial reconstruction, orthognathic surgery, distraction, and cranial reconstruction. The agreement with Materialise will enable us to continue to bring more personalized solutions to the marketplace furthering our ability to improve patient care.”
Based on a CT scan of the patient’s skull, surgeons will be able to design the titanium 3D-printed implants “tailored to the exact shape and size requirements determined by the patient’s condition. The implants can be either a single piece or multiple pieces joined by standard cranial and craniofacial fixation systems, and can be made from either pure titanium or PEEK Optima-LT (polyetheretherketone),” according to 3Ders.org. Surgeons will use DePuy Synthes’ PROPLAN CMF virtual surgical planning software, which comes with the TRUMATCH solution.
“At Materialise, we strive to make medical 3D printing accessible to every researcher, engineer, and clinician, through an open platform of software and services that help customize patient treatment,” stated Materialise Founder and CEO Wilfried Vancraen. “Together with DePuy Synthes, we have successfully enabled better surgical outcomes through surgical planning and patient-specific guides, and are proud that this new collaboration will now empower even more CMF surgeons to discover the benefits of 3D-printed, patient-specific implants as well.”
Image credit: DePuy Synthes
