Life Science Chief Quality Officers Are Redesigning Quality For The 21st Century

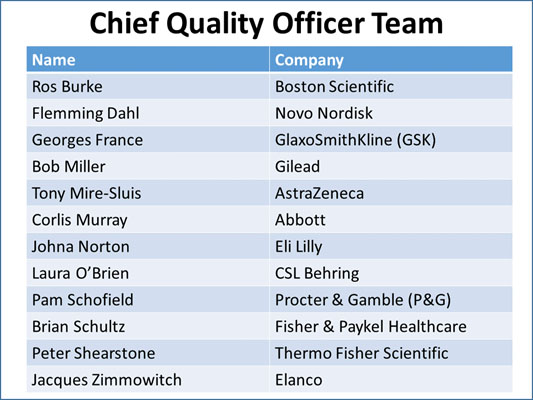
A team comprising a dozen chief quality officers (CQOs) representing the medical device, pharmaceutical, animal health, and consumer healthcare industries is pooling its collective experience and wisdom to redesign the role of quality in the 21st century, with input from the FDA and other regulators.
Research showing that companies in these industries continue to make the same mistakes, despite programs in place to generate corrective and preventive action plans (CAPAs), has driven this team of senior quality executives to completely re-examine their quality models. This retrospection is enabled by conversations with regulatory agencies indicating that they are open to new models in which quality is more integrated with other business functions.
At the FDA/Xavier PharmaLink conference at Xavier University in Cincinnati in March 2019, a subset of the CQO team presented its vision of quality in the 21st century, discussing:
- The challenge of maintaining quality across the product lifecycle
- The complexities of leading a global enterprise with many products, languages and cultures
- The analytical and artificial intelligence (AI) tools needed to analyze exponentially increasingly large data sets and become predictive rather than reactive
- Increasingly complex manufacturing and quality systems and the need to work with regulators and system providers to ensure understanding and compliance, and
- The concept of a quality culture that must be embedded in the organizations and the potential for the quality role to transition from one that oversees to one that mobilizes enterprise-wide quality effectiveness.
Defining Quality For The 21st Century
Xavier Health director Marla Phillips assembled the CQO team in April 2018. The team makeup was consciously designed to get the most thorough, in-depth thinking and solutions from industries where quality departments operate similarly.
First, the CQO team solicited feedback from peers in other functional areas of their organizations regarding their view of the quality organization and its contribution to the company. Responses indicated that the quality function impedes the business, does not understand the business, overcomplicates processes, needs to improve its ability to gain collaborative buy-in, and must be able to articulate the business case for quality (in terms of financial impact
Taking this and other feedback and information into account, the CQO team produced a draft definition for the role of quality for the 21st century: “To optimize patient health and business success by mobilizing enterprise-wide quality effectiveness grounded in science, data, stakeholder awareness and regulatory intelligence.”
Phillips explained the importance of each element of the definition.
“Business success is an important element, because if we do not have a successful business, then we do not have a product,” she said. “How can quality affect the business success? It is not by making decisions because of the pressure from manufacturing. That was the old way of thinking, and that is why quality’s involvement in business aspects was viewed as a conflict of interest.”
“Business success is an important element, because if we do not have a successful business, then we do not have a product. It used to be thought that, if Quality was aware of the business, then there would be pressure to release unsafe products -- it was considered to be a conflict of interest,” she continued. “However, quality can affect business success by removing obstacles to innovation while ensuring systems are in place to strive for right the first time, ultimately reducing risks to the patients we serve."
The team aims to optimize patient health and business success by “mobilizing enterprise-wide quality effectiveness,” as opposed to co-ownership. Proposing that it wants its cross-functional peers to co-own quality would “kind of indicate that they don’t, and maybe they are not bought in,” Phillips explained. “They may not take that very well. It could come across in a way that we are not intending. Instead, we are looking in the mirror and recognizing that we need to create streamlined systems and processes that allow the total enterprise to implement quality in an effective way.”
The team also discussed the need to be agile and to proactively keep pace with innovation.
“Too often, the quality organization is afraid to change, since it requires interpreting the regulations in a new context. However, it would be irresponsible for us to keep innovation from our patients if we have technology that could improve their lives,” explained Phillips. “So how can we mobilize this enterprise-wide quality effectiveness so that it is a valuable tool that drives patient health and business success? The way we can do that is to be sure it is grounded in science, data, stakeholder awareness, and regulatory intelligence. Each of those is key.”
Understanding the goals and metrics of cross-functional peers will help inform quality regarding what they are trying to accomplish, as well as identify areas of conflicting priorities and areas of synergy.
“The last piece is regulatory intelligence. We believe that, no matter what the role of quality in the future could be, it will probably still involve the quality organization bringing regulatory intelligence into the organization,” Phillips maintained.
“With so many emerging markets coming up with their own version of the regulations that are just slightly different from everybody else’s, the organization needs eyes and ears finding and bringing that information into the company. This includes regulations, agency expectations, best practices and successful practices from our peers.”
Maintaining Quality Across The Product Lifecycle
A panel of the CQO members present at the March conference — including Procter & Gamble Global Quality Assurance VP Pam Schofield; Elanco Global Quality VP Jacques Zimmowitch; AstraZeneca Global Quality Head Tony Mire-Sluis; and Gilead Quality Senior VP Bob Miller — discussed the team’s progress to-date.
Procter & Gamble’s Schofield discussed the challenge of maintaining quality across the lifecycle of a product, from development through new iterations.
“Most of our products are global. There are many market regulations that literally change on a daily basis. We are getting pressure for innovation and agility. With all of those constraints, it is really challenging to ensure end-to-end functional groups or disciplines stay in tune with those expectations,” she said.
Schofield pointed out that new products, opportunities, or line extensions could be developed, but “if you have not kept up with the regulations, you could find yourself out of compliance or find that you are holding up the business at the eleventh hour, because you discover the issue either before you send your package to FDA or you launch.”
She stressed the importance of ensuring that knowledge is preserved and transferred and internalized by everyone from R&D through manufacturing. “I view quality as the backbone of that. We are in each of those places with our partners, but we need to be one with the knowledge,” Schofield said.
The CQO team also discussed how to involve cross-functional end-to-end peers in each of their companies. Procter & Gamble has formed teams in each of its business units, collectively called “the seamless technical community” and comprising individuals from R&D, manufacturing, and quality.
“We are trying to drive that knowledge. We are also developing a digital system to help. It cannot replace the knowledge, but it certainly can be an enabler to having knowledge captured. We need a way to capture the data and access it so that we are not redoing things over and over again, but so we have the knowledge and are building forward,” Schofield explained. The CQO forum is discussing how each member company is doing this and how they can benefit from sharing their efforts.
One of the challenges, Schofield said, is to help R&D understand what manufacturing needs from them. “R&D generally does not understand what it takes to manufacture and deliver the product. They do a lot of innovation and a lot of good work. But what gets transferred to the manufacturing plants and to the labs, including the methods, tends to be focused on R&D and is not really commensurate with what needs to be true today to release product in a real-time fashion.”
Having R&D understand what is needed from them, Schofield continued, “is critical, because in a manufacturing environment there is a time constraint and everything is compressed.”
AstraZeneca’s Mire-Sluis commented, “Product lifecycle can actually have two different meanings. It can be as Pam was describing – from R&D through commercial manufacturing. Or it could be actually how you make a product, from procurement to the raw materials to the manufacture, release, and distribution. We need a quality organization that touches all of those functions.”
Elanco’s Zimmowitch pointed out that, in some companies, R&D and manufacturing exist as silos.
“Over the past 28 years, I saw the R&D silo in place with the results you are talking about. A process was given to manufacturing and it was like fitting a square peg into a round hole. It was not working. I have seen a lot of change in the last ten years — when we stopped talking about different objectives for R&D, manufacturing, and the business, and began having a common objective, which is to have a reliable supply of quality product. We have a business case for that. We align everyone’s objectives to that.”
Leading A Global Enterprise With Multiple Products, Languages, and Cultures
During the presentation, Phillips asked, “What if, as an industry, we could establish and maintain a simplified quality system that transcends leadership styles, risk tolerances, and human error in a way that ensures the protection of our products and the patients that is not dependent on individuals?”
The challenge, and an area the CQO team is looking at, is how to remove that variability.
Gilead’s Miller commented, “We went from [operating in] 20 countries to 135 countries. How do you set those common standards? We decided to keep it simple. At the end of the day, there are probably 10 or 12 key quality systems. If we get those right, we are probably in really good shape. Do not think that everything has to be the same across the board. But for key aspects – for example, escalation management, deviation management, complaint management, and training – we decided to start there and have the conversation.”
“We have also talked about having our own standard – a Gilead standard. It will be at least as good as the local standard. That is the first thing we did.”
Schofield noted that P&G faced a similar situation in terms of portfolio diversity and the transformation of its quality management system a few years ago.
“We have a limited number of core systems. If you try to do everything and institute it globally and mandate it, it is really challenging. We follow the local laws, as well as our P&G standard. If the country standard is higher, we follow it. I would echo what Bob said – really trying to have a system that can be utilized and internalized by the people is important.”
Increasing Amounts of Data Require More Analytical Capability
Elanco’s Zimmowitch described the challenge of implementing the tools needed to support product quality assurance, beginning with three observations:
“[First], the quantity and complexity of all the data and information that we are exposed to have been growing exponentially in the past few years. And it continues to accelerate. It has become an overload of information. And it will get even more complicated. We see, for example, all of the issues that a lot of us are having in terms of ‘connected care’ programs, where we are receiving patient feedback much more directly. For a lot of us, these are coming from all over the world, in different formats and languages.”
Second, organizations must contend with key stakeholders “whose expectations are changing and evolving very quickly,” Zimmowitch said. To satisfy those expectations, the quality organization “needs to be faster, more agile, and more proactive. We need to be able to detect signals and trends earlier. We can use that information to mitigate or avoid potential customer impact.”
Third, industry tools and the way companies look at data and information “have not really evolved at the same pace as our needs in the past 15 years. Our needs today are far beyond the classic Excel spreadsheets being used in a lot of places to manage complex information,” Zimmowitch said.
He reported that the CQO forum has taken one key quality system process — customer complaints — and asked, “what if we, as an industry, could detect a trend in customer complaints, and identify the root cause in minutes instead of weeks, and not have to read hundreds of complaint reports?”
Thus, Zimmowitch asked his development team and IT group to explore text and cognitive analytics as a solution. He noted that the proof-of-concept phase, applying the process to the pharma space, showed that trends and root cause could be identified — in some cases — in hours or minutes, “when the manual human process was taking weeks, at best, to perform the same identification.” Elanco plans to implement the system worldwide.
“I think there are opportunities to be more proactive and efficient and detect performance shifts or drifts in spaces such as complaint management root cause analysis, pharmacovigilance, environmental monitoring, or anywhere where we manage tons of data,” Zimmowitch said.
Barriers To Innovation And The Rapid Pace Of Change
AstraZeneca’s Mire-Sluis focused his discussion on the rapid pace of change and barriers that may be presented by available work force, regulators, and suppliers of software solutions.
“At our company, we were talking about electronic batch records. Everybody, we think, wants electronic batch records. It is going to save loads of signatures and loads of paper. But when we talk to some pharma manufacturing operators, they tell us, ‘I like my paper. I can follow it. It is not scary,’” he said.
The lesson, Mire-Sluis said, is to understand that not everybody embraces technology. In addition, “Our industry may not have a workforce that is capable and willing to work in areas that involve automation, electronic systems, advanced analytics, artificial intelligence, etc.” This type of change requires time, education, socialization, engagement of those impacted, and lots of communication.
Further, “if we are going to have some new technology that needs approval, how do we partner with the regulators to make them comfortable with some of the new technologies? They are not in our factories; they are not working with that technology,” Mire-Sluis said.
The new technologies, for the most part, come from system providers. System providers often do not understand GMP requirements, such as Part 11 compliance, audit trails, or electronic signatures, Mire-Sluis maintained. “How do we work with them from the earlier stages so, when the technology comes to us, it is in a much better place than us having to work backwards with them? I think we all eventually want to implement AI. [So,] why not learn it as an industry, instead of in our own silos? That is one thing we are trying to do with the CQO team.”
Mire-Sluis also pointed to the CQO team’s efforts to create university-level minors, majors, and undergraduate degrees in quality.
Quality Culture And Moving From Ownership to Oversight
“The concept of a quality culture is something that has to be embedded on our organizations, which is a change,” said Gilead’s Miller of one topic area the CQO team is examining. “Rather than quality owning everything, the quality role may transition from an owner to an oversight role.”
“Another aspect is, what is the role of the quality leader in the future? We all hire people like ourselves. That is part of the problem. We want people who think like we do. That does not allow us to change.”
Miller also noted how difficult the quality role can be.
“Someone once said to me, ‘When a quality person comes into a meeting, they are not coming in to give good news. It is usually because something has happened, and we need to talk about where to go from here.’ That becomes a tough job,” he said. “People use it as a springboard to go somewhere else. It is great for us if they go somewhere else, because they take the quality mindset with them,” but we also need some to stay in the organization as quality professionals.
Miller explained that the skill sets quality professionals will need will look different in the future. New skill sets would include the ability to tell a story that explains why something needs to be done. Another skill set will be data analyst, a role for which Miller hired an individual previously employed with a major tech firm. “It will bring a different mindset because they may look at data very differently than we look at data.”
Other skill sets the quality professional of the future may need include teacher, mentor, and root cause analysis specialist, which Miller characterized as very specific skill sets. He maintained that succession planning will be very important in the quality organization, specifically because of the skill sets that will be required.
“We need to instill that quality is beyond compliance… That is not well-understood by a lot of our business partners. That is very important,” Zimmowitch added. “We look at the supply chain of the product, but there is also a supply chain for information and data. Think about labeling claims, promotional material, and so on. If we elevate the discussion that quality is not only about product, in my experience, people listen a little bit more.”
Future Plans
The CQO team’s future plans include continued reexamination of the role of quality, alignment of cross-functional metrics, identifying critical HR processes to support sustainable and impactful change, and creating the next generation of quality professionals, Phillips reported.
“We have people who work for the 12 chief quality officers dissecting the role of quality today versus the role quality should have,” she said. They are looking at the activities in which quality is involved, and attempting to determine whether it makes sense for quality or another area to lead each activity. “What support, what tools and information would they need to make that happen, and how would we measure the success?”
Graduate students serving the team are looking at the top three metrics tracked by quality’s cross-functional peers. The students are looking for commonalities, areas where a quality metric might shut down a metric that a cross-functional peer is trying to achieve, and vice versa. They also are using the exercise to inform quality about what is important to its stakeholders.
The CQO team also is looking at critical HR processes that can support sustainable and impactful change. “If we are going to shift the way quality is operating, it cannot just be assigned to someone else. There has to be a program that includes objective setting, metrics, and performance appraisals that are all based on the behaviors we are trying to drive. “
The dialogue continues at the FDA/Xavier MedCon conference April 30 – May 3, 2019 at Xavier University in Cincinnati. CQOs presenting on their efforts and available for networking include Georges France, VP & Global Quality Lead, GSK; Corlis Murray, Senior VP, Quality Assurance, Regulatory and Engineering Services, Abbott; Brian Schultz, VP of Quality and Regulatory Affairs, Fisher & Paykel; and Peter Shearstone, VP, Global Quality Assurance and Regulatory Affairs, Thermo Fisher Scientific.
About The Author
Jerry Chapman is a GMP consultant with nearly 40 years of experience in the pharmaceutical industry. His experience includes numerous positions in development, manufacturing, and quality at the plant, site, and corporate levels. He designed and implemented a comprehensive “GMP Intelligence” process at Eli Lilly and again as a consultant at a top-five animal health firm. Chapman served as senior editor at International Pharmaceutical Quality for six years and now consults on GMP intelligence and quality knowledge management and is a freelance writer. You can contact him via email, visit his website, or connect with him on LinkedIn.
