New Development In Heart Valve Patent War Between Medtronic, Edwards
By Joel Lindsey
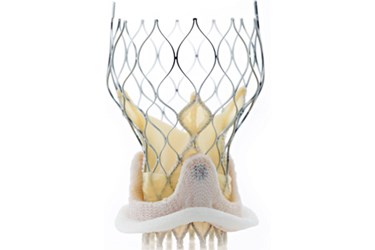
Late last week, Medtronic announced that the European Patent Office (EPO) invalidated a patent held by Edwards Lifesciences that had previously prohibited Medtronic from selling its CoreValve transcatheter aortic valve in Germany.
“Medtronic is very pleased with this ruling as it will ensure that patients across Europe who need aortic valve replacement will have access to this lifesaving therapy,” John Liddicoat, senior VP of Medtronic, said in a press release issued by the company.
Last August, a German court ruled in favor of Edwards when it found Medtronic’s CoreValve product to be in violation of an Edwards patent on a similar type of transcatheter aortic valve replacement (TAVR), known as the Spenser patent.
This initial ruling was later called into question by the EPO, which ultimately declared the entire Spenser patent invalid.
According to a news article published by the Star Tribune, Medtronic and Edwards are the two biggest players in the TAVR industry.
The two companies have also recently been embroiled in a patent litigation case in the United States, after a federal court jury in Delaware ruled that Medtronic’s CoreValve device infringed on an Edwards patent. In the case, the court demanded that Medtronic pay $393.6 million in damages, but Medtronic plans to appeal this verdict, the Star Tribune reported.
TAVR devices, like those manufactured by both Edwards and Medtronic, are designed to provide treatment for patients who suffer from diseased aortic valves but who cannot undergo traditional therapy. TAVRs make it possible for surgeons to implant a new valve into a patient’s heart via a catheter inserted into either an artery in the leg or a small incision in the upper body.
In many cases, aortic valve replacements require surgeons to cut open a patient’s chest, but for patients too sick or weak to survive such an invasive procedure, TAVRs present an alternative mode of treatment.
The EPO’s most recent ruling in the Medtronic-Edwards case opens up new markets for Medtronic’s CoreValve, a product that was initially approved for sale in Europe in 2007.
This past January, the U.S. FDA approved CoreValve for use in the treatment of patients at extreme risk for open-heart surgery. Medtronic also hopes to obtain U.S. clearance to sell the device to patients deemed high risk sometime later this year.
Image Credit: Medtronic
