Researchers Develop First Fully Implantable Fetal Pacemaker
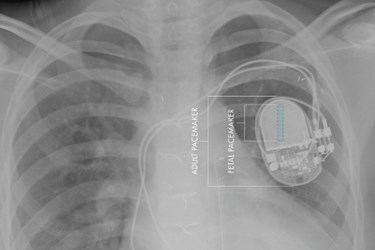
Scientists have developed the first pacemaker designed specifically to be implanted in fetuses with complete heart block. The device was recently granted a humanitarian use device designation by the FDA, and its developers expect the first human implant to take place in the near future.
Complete congenital heart block occurs when a defect within the heart’s electrical system causes the fetus’ heart to pump slowly and ineffectively. Though the condition can be accurately diagnosed, attempts at intervention have thus far been unsuccessful, and the pregnancies usually end in miscarriage or induced premature delivery.
According to a recednt Children’s Hospital Los Angeles (CHLA) press release, surgeons have tried to use standard pacemakers in utero by implanting wires into the fetuses that are connected to an external device. These efforts failed, probably because fetal movement often detached the electrodes.
Now, researchers at CHLA and the University of Southern California (USC) have developed a device that fills this unmet critical need. “Up until now, the pacemaker devices that have been used to treat this condition in a fetus were designed for adults. We have lacked an effective treatment option for fetuses,” CHLA pediatric cardiologist Yaniv Bar-Cohen, M.D., said in the press release.
In comparison, the CHLA-USC team’s microdevice is fully implantable and designed to withstand normal fetal movement.
“Building on our experience of using microfabrication techniques to create biomedical devices, we have developed a micropacemaker small enough to reside entirely within the fetus,” said Gerald Loeb, USC professor of biomedical engineering. “This will allow the fetus to move freely without risk of dislodging the electrodes.”
Last year, the team published a schematic of the device and its preclinical results in rabbit models in Annals of Biomedical Engineering. After another two years of testing and optimizing their design, the team published again in Heart Rhythm.
They also applied for and received a humanitarian use device designation from the FDA.
Last year, in a keynote address at the Second Annual Pediatric Surgical Innovation Symposium, FDA Commissioner Margaret Hamburg spoke about the highly complex problem of scaling medical devices for use in fetuses, infants, and children and detailed the unique set of challengers.
According to Hamburg, the design of pediatric medical devices “requires taking into account such factors as unpredictable growth and development, hormonal influences, anatomic and activity levels.” Changes in size and thickness of material used, she added, could also affect the integrity of the device’s function.
The CDC estimates that 40,000 American children are born with congenital heart defects, which are the leading cause of defect-related illness or death among infants.
Researchers said in the press release that 500 American pregnancies per year could benefit from this device.
According to Ramin Chmait, director of the CHLA-USC Institute for Maternal-Fetal health, “We now have a pacemaker that can be implanted in utero, potentially without harm to the fetus or the mom. This novel device provides a real opportunity to prevent miscarriage and premature birth in babies affected with these abnormalities.”
Image credit: Children's Hospital Los Angeles
