Key Design Considerations For Digitally Connected Injectors
By Jason Song, SureMed Technologies

Digital health has become an integral part of clinical trials and the commercial patient support ecosystem. In the past two years, there has been a significant transformation in the way clinical trials are conducted, as well as how healthcare providers support patients and patients’ expectations around their illness management. With COVID-19 and the public health lockdowns and social distancing measures, clinical trials leaped from traditional in-person interactions between investigators and trial participants to a more virtual setting with an open embrace of digital health and telemedicine tools. With social distancing and challenges around in-person visits to clinics, insurers, healthcare providers, and patients have also embraced and championed the use of digital and virtual tools. Today, doctors are more accepting of reviewing a patient’s heart monitor data generated by the patient’s Apple smartwatch. In fact, talking to healthcare providers, doctors, and nurse practitioners reveals that digital tools provided more value and unexpected benefits, such as the ability to see a broader view of patients’ health situations over time between visits.
Pharmaceutical companies have also seen the benefit of integrating digital capabilities within clinical trials. First, no longer is recruitment hindered by geolocation and proximity to trial sites. In addition, for the past five or so years, pharmaceutical companies have seen the benefit of integrating digital tools and technologies as part of a digital patient support ecosystem. The tools and technologies allow for better patient engagement and treatment support that translate to stronger brand position, real-world evidence, and deeper patient insights. In the case of inhaled products such as Symbicort, digital connectivity through inhaler add-ons or integration has demonstrated improvement in patient disease management through improved adherence and compliance. In addition, data from connected inhalers, such as the Turbohaler from AstraZeneca, have generated real-world information, such as the number of user days on treatment, as well as helping to identify predictors of asthma attacks and/or disease exacerbations.
Today, we see wide adoption of digital inhalers, and many newly launched inhaled products have digital connectivity capabilities that integrate them into a patient support digital ecosystem. This, unfortunately, is not the case in the injectable drug delivery device space. Today’s injection drugs are still being developed and launched without a true connected device element to track adherence and compliance. Worse yet, while many inhaled products are going into clinical trials with connected inhalers to help with trial adherence and compliance as well as support clinical trial endpoints, there are no connected injectors going into clinical trials. As connected drug delivery devices require clinical trial data to support their use and benefit before they can be submitted for regulatory approval, this means we most likely will not see connected injection devices, such as prefilled syringes, autoinjectors, or needle safety devices that are widely used for biological drug products, for some time. The exception is in the diabetes space, where we have seen a wider advancement in pen or multi-dose injector digital connectivity, as well as one or two products that utilize expensive syringe injection aid/assistance devices, such as the syringe injection assistance device for Betaseron, whereby the user loads the syringe onto a battery and motor operated injection assistance device to help with injection.
With the wide success of connected inhalers, it raises the question: Why aren’t there any successful connected injectable devices despite the many companies offering connectivity accessories for injector devices? To answer this question, we will have to dive into what makes a successful connected drug delivery device.
Make Sure Your Connected Injector Fits Within A Digital Ecosystem
A successful connected drug delivery device must first and foremost fit within a digital ecosystem, whether it is a clinical trial ecosystem or a commercial patient support ecosystem. The connected drug delivery device is a special case, as its role within both the clinical and commercial digital ecosystem is to confirm that the patient has taken their medication. As such, it requires the same connected device to be used in the commercial environment as was used to demonstrate adherence and compliance in clinical trials. In the case of a connected drug delivery device, any device data captured beyond whether the medication was administered or not provides no additional added value and will oftentimes throw the digital ecosystem out of balance, resulting in a detrimental hindering effect. A digital ecosystem can be viewed like a watch; each component must serve its purpose exactly, no more and no less. When one piece over- or underperforms, it will throw the system out of balance, just as an incorrect gear in a watch will render the watch inaccurate and, over time, nonfunctional and abandoned.
Similarly, additional information or data gathered by a digital device beyond its purpose can create unnecessary confusion and problem for the patient, user, and/or parties involved with treatment management. Using another analogy, the speedometer on a car serves only one purpose and that is to tell you how fast you are driving. It needs to function exactly as intended for the driver. Capturing too much information, such as how bumpy the road is, how straight you are driving, the frequency of acceleration and brake usage, etc., will not help but will only distract the driver from the critical speed information that the driver needs to maintain safe driving. This is why, at the moment, cars have hands-free call features but no features to support texting or IMs.
To this end, I will start off by first analyzing why connected inhalers and connected insulin pen injectors, by and large, have been successful, while connected syringe and autoinjector connectivity solutions by large have missed the mark and have largely been rejected and eventually abandoned by pharmaceutical manufacturers.
Connected inhalers and connected insulin pen injector add-ons and solutions have been successful because those solutions provide the balanced data to fit within their respective digital ecosystems. And in doing so, they demonstrate their value in helping patients stay adherent to their medication regimen. Connected insulin pen injectors have been proven to help patients maintain and keep their H1Ac levels in check and, therefore, help slow disease progression and minimize health complications that are associated with uncontrolled diabetes. These devices, combined with artificial pancreas systems, have revolutionized diabetic care management and patient enablement. The same goes for connected inhalers. Connected inhalers that only capture medication usage information have fit perfectly within the asthma digital ecosystem by providing key usage information and have been proven to increase days on treatment and reduce asthma attacks for patients.
The commonality between inhalers and pen injectors is that they are both multidose devices. An inhaler can be used for up to a month. Pen injectors utilize cartridges with formulations designed for repeat use. Each time the pen injector user uses the device, they attach a new insulin needle. The pen injector and its drug typically are designed for up to a month of repeat use and are replaced afterward, similar to an inhaler. In such a scenario, having Bluetooth connectivity for both the connected inhaler and connected insulin pen injector makes complete sense. The user will pair the inhaler with the add-on or pen injector with connectivity to their smartphone whenever they replace their medication. The smartphone, with its processing capacity and user interface, is the focal point for patient end-data gathering and the central patient interface. For connected inhalers and pen injectors, connecting the user’s smartphone with the connected device does not take place when they need to take their medication but instead occurs when the user replaces their medication, typically at a scheduled time of the month. As such, performing the pairing operation and checking for proper connectivity between the inhaler/pen injector and the smartphone does not take place during the stressful act of taking the medication. On the other hand, prefilled syringes, prefilled syringes with needle safety devices, and autoinjectors are single-use ready-to-use devices designed to be discarded after use. Yet what we have seen is that many connectivity solutions put forth for these single-use devices follow the Bluetooth connectivity model of multi-use devices like inhalers, without taking into consideration the patient’s use situation or the disposable/single-use nature of the device. As a result, those solutions, if assessed at all, are guaranteed to be rejected by pharmaceutical manufacturers.
Above, we discussed the connectivity technology used. The choice of wireless communication technology is one of the essential early considerations for a connected device. It needs to be selected based on the device’s intended use situation, as well as with the end user in mind. We’ll continue with the device’s use situation just alluded to and will utilize our SureMed Tech’s product lines to demonstrate why, when selecting the wireless communication technology, we must also take a holistic view of the end user for the developed connected device to be successful.
Use NFC, Not Bluetooth, For Single-Use Disposable Injectors
We at SureMed Tech believe disposable connected injectors such as digital syringes and autoinjectors need to utilize NFC as opposed to Bluetooth, based on the product’s use situation. Only fixed-dose drug products are placed in disposable injectors such as prefilled syringes or autoinjectors as they are injected in full and disposed of afterward. As such, the patient will only take those products out of refrigeration at the time of use. If the digital solution for these disposable injectors is built around Bluetooth, as many failed injector digital connectivity products have been in the past, following the connected inhaler model, what we are essentially doing is complicating an already complicated process for the user by asking them to perform Bluetooth pairing and check for successful Bluetooth pairing while they are trying to keep straight all the medication steps while mentally preparing for the injection. This adds to the user’s stress and anxiety. With NFC, there is no need to perform Bluetooth pairing; it is ready to go for the patient. Their preparation steps stay the same as before, and only after completing the injection do they just tap their used injector device to their smart phone to record that they have taken their medication, similar to tapping their phone to pay at the grocery store or tapping their phone to open a hotel door.
While there may be other wireless communication technologies that allow for quick pairing, such as QR codes and smartphone camera mode, those still introduce additional steps and adds complexity to an already complex process. Over time, the user will find it cumbersome to use the smartphone camera. We found in our interaction with patients that over time, patients feel it is more like proving that you have done your homework as opposed to being proactive with their medication tracking. Second, except for online banking, we are not in a habit of using the camera feature to do anything other than taking pictures or videos.
Taking into account our everyday experience, NFC will prove easy to adopt and most beneficial to the patient. The reasoning is that NFC is widely used, from credit cards to hotel room access, company IDs and access badges, metro and train station passes, and smartphone tap and pay functionality. As such, NFC and its tap-to-use is ingrained in our everyday behavior. This is important when building digital products as we need to utilize behaviors and technologies that are entrenched in our daily lives to minimize adoption challenges and ingrained behavior incompatibility. Imagine if a digital product is built on ZigBee wireless communication; it may be the best product and ZigBee may provide it with the best wireless communication for its need. Nevertheless, since no product on the market uses ZigBee, it would be a hard ask for the end user to learn how to use ZigBee just to use your product.
Consider The Needs Of Your Users
While matching the right wireless technology to the end use situation and the end user’s preconditioned experience and behavior is an essential step, in addition we must also consider the various stakeholders needs within a digital ecosystem. In the case of healthcare providers and doctors, as mentioned before with the watch and car analogies, for a connected drug delivery device, the balance of information captured and the connectivity purpose should always be considered. Over the years, we have seen many products try to do more than needed and, in doing so, rendered the connectivity solution flawed and unusable as those additional features often result in products that provide distracting and sometimes counterproductive information. From all of our stakeholder interactions, we found that the purpose of the connected drug delivery device, be it accessory or integrated add-on, must only do one job and that is to provide a way for the patient to confirm to the digital ecosystem that they have taken their medication and nothing more. The digital ecosystem has other integrated solutions/products to provide the reminders, prompt for diary entry in the case of clinical trials, etc. Too often, we seen connected injector solutions try to perform more than is necessary, such as having a pre-injection login step that then walks the patient step by step through how to use the device or that provides a digital set of the product name, batch record, and/or expiration data information for the app to asks the patient to confirm against the syringe label. What’s worse is that we have seen some products that force the patient to go through a step-by-step walk-through of the device preparation and injection steps with each step requiring the user to tab a “next” icon on the smartphone that appears only at the end of each step tutorial.
This is all happening during or concurrently with the patient’s preparation and injection process, adding additional unnecessary anxiety and stress on the patient. In addition, walking the user through the injection steps should not be a forced activity every time the user gives themselves an injection. This is an onboarding feature that helps familiarize and/or remind the user of the administration steps. After the user has had experience, this should not be repeated every time. It would be like forcing the user to watch the same TV ad over and over and over. Instead, as we have advised countless pharmaceutical clients, this should be an optional feature, such as a help icon that the user can go to if they want a refresher on the steps association with taking their medication.
This gets into the other problem we have seen with failed connected injector solutions. The selection of connectivity technology is critical. To distinguish between an un-injected and an injected syringe or autoinjector, there are a number of designs that utilize a switch-based NFC design that, prior to injection has one signal and after injection has another signal. The problem here is that this design requires a manual switch interface (custom-designed atypically shaped finger flange that must interface with custom-designed atypically shaped plunge rod, for example), resulting in a complete change in product look and feel. We will discuss this shortly in this article. In addition, this also creates the unintentional problem that it requires the user to interface the device with the smartphone app once beforehand when the patient is preparing and getting ready to administer the medication and again after medication have been administered. This adds additional user steps and complexity.
Those designs are little better than Bluetooth as they still require the user to preregister the device – basically, pairing of the device to the app – followed by a second register or tap of the device to the smartphone after use. First, this creates a lot of room for user error and confusion, and this again asks the user to remember and perform additional tasks and procedures while they are preparing and getting ready for an injection. In addition, this is counterintuitive to our ingrained behavior. We do not tap our phone twice to pay at the cash register. We do not tap our ID badges twice, once to initiate and another tap to unlock the door. We are preconditioned through our everyday NFC-based products that one tap is enough. So, oftentimes we see patients with the aforementioned failed NFC devices at first tap the device to register/pair it with the smartphone, but then they do not tap the device again after injection. When asked why, the most common response is they forgot or that they thought one time is enough just like their badge at work. Similarly, when asked about the pre-use tap/pairing and post-use tap, participants typically find it cumbersome. Many participants, when asked what they think of using the two-tap NFC design in their daily lives, indicated they would most likely forget to do the first tap. When asked further, the reason given by a large number of participants is that with all the preparation, they would most likely forget to do the first tap and remember to tap the device to the smartphone when the injection is done.
Therefore, for adherence and compliance, it is essential to use a technology that only provides a signal after the device is used/after the medication is injected to confirm that the device has been used. And this is what we have done at SureMed Tech. We have developed our proprietary NFC technology whereby the device does not transmit any signal until it has been used, as shown in Figure 1 below. This way, it provides a much more reliable medication use confirmation. In patient surveys, we found that users prefer this type of connected device, as it is in line with their daily experience with NFC technology (badge access, tap to pay, etc.). In addition, we found that patients prefer a device that transmits signal only after use because it keeps things simple and straightforward for them.
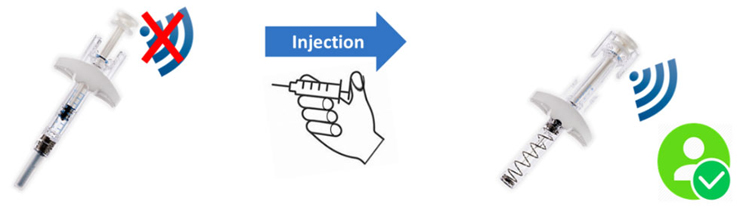
Figure 1
What we also found is that users prefer to have an NFC signal available only after injection to add to their treatment record because after completing the injection, that’s when they can think about things such as adding a confirmation to their medication history. Many of our clients have embraced our signal only after using the connected injector products, as they see this as a true adherence and compliance digital confirmation technology that, unlike prior solutions, will help them monitor their medication adherence and compliance during a clinical trial and, later in the commercial patient support ecosystem, provide reliable adherence information correlating real-world data to support treatment efficacy and patient benefit information.
A key factor for a successful connected device is user experience and device form factor, or how the device presentation looks and feels. The adaptor type of connected accessories for injector devices typically changes the overall look and feel of the original device, which we have found to be unacceptable to end users unless it provides additional user benefits such as having ergonomic features to aid in administration.
Maintain The Look & Feel Of The Original Unconnected Device
Connected injector solutions today (with the exception of SureMed Tech’s) will result in a completely different-looking end product than the original injector product. This is especially so with the recently developed connected syringe, whereby the backstop and plunger rod are a new design with different shape and form, rendering the prefilled syringe a completely new device presentation with a completely different look from the predecessor. As a result, this will also require its own separate packaging and package inserts as well as new design and development work and new regulatory submission. This is unacceptable to many users expecting the connected form of the device to be simply an extension of the previous device that maintains the same look and feel as before. Pharmaceutical companies also want the connected device to be the same look and feel as the original device and do not want the additional cost and time for development and implementation of new manufacturing lines or the hassle of having to introduce another new presentation to the brand. Due to these reasons, it is important for connected injector solutions to be able to maintain the same or similar look and feel of the original injector device.
We have taken this to heart when developing our eConnect products. In Figure 2 (below), in the case of our ePlunger Rod, the digital plunger rod is of the same look and feel as the original plunger rod. The ePlunger Rod is designed so that the pharmaceutical manufacturer can easily substitute the original plunger rod with SureMed Tech’s ePlunger Rod to digitally enable the prefilled syringe or needle safety device. This way, the user experiences the same look and feel with the new digitally enabled prefilled syringe or needle safety device.

Figure 2
Similarly, as shown in Figure 3 (below), our eSleeve follows the same mindset in which the thin cover sleeve fits right over the autoinjector and does not change the look and feel of the autoinjector. Our user opinion studies found it to be a key adoption point for end users.

Figure 3
From our pharmaceutical partners, we have found that our approach to keeping the same look and feel so that the ePlunger Rod can easily replace the traditional plunger rod in pharmaceutical manufacturing and that the eSleeve can easily integrate as an added manufacturing step within the existing manufacturing process, making the incorporation of the digital solutions much easier and less costly.
Another aspect of a successful connected solution is its ability to provide value add. Value add can be in the form of ergonomic adaptions, as in the case of our eSleeve design, for better hand grip to further strengthen the pharmaceutical product’s brand, or it can be in the form of convenient life cycle management, as in the case of our eCap, shown in Figure 4 below.

Figure 4
In all the cases, we believe the future of connected solutions is platform consideration. To date, only our line of connected solutions addresses all injection systems (prefilled syringe, needle safety device, and autoinjectors) from a platform perspective. From the eCap solution in Figure 4 to the ePlunger Rod and eSleeve solutions shown in Figure 2 and 3, SureMed Tech’s eConnected products communicate with the smartphone app the exact same way as shown in Figure 5 below. This allows for seamless transition of patients between a prefilled syringe and an autoinjector. This has proven to be a needed and welcomed consideration by our pharmaceutical partners.

Figure 5
Handle Post-approval Changes Smartly
Sometimes, products are already on the market and significant changes will require extensive development; as such, any family of connected solutions should take this into account and minimize the impact on existing market device presentations. Take SureMed Tech’s eCap as an example. For prefilled syringes that are already on the market, changing the device may not be an option. Therefore, the eCap is designed to go onto an existing prefilled syringe’s plunger rod as a clip-on element. This minimizes the design change associated with the existing device.
A successful connected device solution must also integrate within existing pharmaceutical manufacturing operations. Changes to existing devices and manufacturing processes should be minimized as much as possible.
Take our ePlunger rod, for example. The ePlunger rod is designed to provide the same patient/end user experience as the existing plunger rod. Unlike other digital syringes that require a completely new backstop and plunger rod, our ePlunger rod is designed to be interchangeable with the existing plunger rod. This also prevents or minimizes the impact on existing manufacturing processes and equipment. The ePlunger rod can simply replace the current plunger rod and the manufacturing process stays the same.
Another crucial element of a successful connected product is that it should impact the pharmaceutical manufacturing minimally. Take our eSleeve connected solution for auto-injectors as an example. The eSleeve is designed to slip permanently onto existing autoinjectors like a thin external ergonomic cover/sleeve. This minimizes both the autoinjector’s form factor and any changes to the user’s experience, as well as allows for easy integration of an eSleeve attachment step within the existing manufacturing process. As an example, the eSleeve can be integrated as a last step in the device assembly process or as part of the packaging and labeling operation.
Finally, the third element that we at SureMed Tech see as key to a successful connected device solution is that the product should be designed to be cost effective. We will not go into discussion on cost here. Suffice it to say, the cost of an ePlunger Rod would not be significantly higher than the cost of the standard plunger rod.
Conclusion
In summary, for a connected injector device, we cannot simply just copy and paste what has been done for connected inhalers. Connected injector devices must be viewed through the lens of the device use situation and the end user/patient, as well as the varying needs of all stakeholders. Your family of platform digital products should be designed taking into account users’ everyday experience beyond medication use to develop a product that:
- utilizes NFC
- provides communication only after the device has been used, so the user just has to tap once after use, in line with our everyday experience and expectations
- provides just the information that healthcare providers and clinical trial investigators want (medication use), thus allowing it to fit within the digital ecosystem
- keeps to the same form and presentation as existing products, allowing easy integration into existing pharmaceutical manufacturing processes
- is cost effective.
If you would like more information or have questions, please reach out to me directly at jsong@suremedtech.com. If you have any other topics or question you would like us to cover or discuss in a future article, please feel free drop a line in the comments section or reach out to me directly.
About The Author:
Jason Song, P.E., is chief technology officer of SureMed Technologies, Inc., a company he co-founded in 2018 that develops holistic novel technologies, products, and services that balance the needs of patients, industry, and the healthcare ecosystem. Previously, he held various technical and leadership positions at Amgen, Eli Lilly, GE Healthcare, Motorola, and Novo Nordisk in a range of areas, including injectable and inhaled drug delivery device development, fill and finish, packaging and assembly automation, biochips, battery development, and establishing new production sites. He holds BS and MS degrees in mechanical engineering, an MS in automation manufacturing, and an MBA.
