ARTICLES BY JOF ENRIQUEZ
-
UK Scientists Invent Camera That Can 'See Through' Human Body9/7/2017
Scientists at the University of Edinburgh and Heriot-Watt University, through the Proteus Interdisciplinary Research Collaboration, have developed a new camera that can image individual photons emanating from fiber optic medical instruments inside the human body.
-
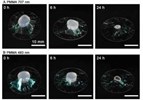
Water-Repellent Nanosheet Wrapper Enhances Biological Microscopy8/24/2017
Japanese scientists have developed a unique water-repellent nanomaterial that can be wrapped around biological tissue to visualize high-quality images for a longer period of time.
-
FDA Clears First RF Ablation Treatment For Osteoarthritis Knee Pain6/20/2017
The U.S. Food and Drug Administration (FDA) has approved the first and only radio frequency (RF) treatment to be cleared specifically to alleviate chronic knee pain due to osteoarthritis.
-
Medical Device Companies Cope With India's Stent Price Caps5/2/2017
Medical device companies Abbott, Boston Scientific, Johnson & Johnson, and Medtronic are trying to come to grips with Indian regulators’ decision to set a price cap on cardiac stents by refiling applications to withdraw certain products, as well as lobbying against such regulation, which they contend increases the risk of introducing new products into the country and places such products out of patients’ reach.
-
Boston Scientific Looks To Keep Up Momentum, Preps Return Of Lotus TAVR Device5/1/2017
Boston Scientific's chief executive says the company intends to continue its momentum achieving top-line growth across all business segments, and is preparing to re-introduce its Lotus device into the burgeoning transcatheter aortic valve replacement (TAVR) market in 2017’s fourth quarter.
-
Zimmer Biomet CEO Confident In Restoring Adequate Supply To Fuel 2017 Growth4/28/2017
Zimmer Biomet CEO David Dvorak told investors that the company will build on gains achieved during the first quarter as the company ramps up production in its Warsaw facility, which is subject to ongoing remediation activities.
-
Edwards Lifesciences Soars On Higher TAVR Sales, Sets Sights On Mitral And Tricuspid Therapies4/27/2017
Edwards Lifesciences reported better-than-expected first quarter numbers behind surging sales of its transcatheter aortic valve replacement (TAVR) devices.
-
Philips Off To Solid Start In 2017, Expects Growth To Accelerate4/26/2017
Philips' HealthTech portfolio powered the company's first quarter performance, and growth is expected to accelerate through 2017, despite weakness in the United States market related to potential healthcare policy changes.
-
Becton Dickinson Diversifies Via $24 Billion C.R. Bard Acquisition4/25/2017
Becton Dickinson (BD) is paying $24 billion in cash and stock to acquire C. R. Bard to move beyond diabetes care and into peripheral vascular disease, urology, hernia, and cancer treatments, as well as to round out its market-leading medication management portfolio.
-
Danaher CEO Expects Acquisitions, R&D To Fuel Long-Term Growth4/24/2017
Danaher Corporation's recent acquisitions, reinvestments, and the Chinese market will continue to help drive long-term growth, according to the company's CEO, Thomas P. Joyce, Jr.