Companies To Watch: Pulse Therapeutics
By Jim Pomager, Executive Editor

Magnetically spinning nanoscale particles may overcome a major barrier to effective stroke treatment — helping clot-busting drugs traverse stopped arteries.
Snapshot
Pulse Therapeutics has developed a mechanical thrombolysis technology that accelerates the delivery of clot-busting drugs to a clogged artery. The Magnetically Enhanced Diffusion (MED) System consists of intravenously administered nanoscale iron particles and a patient-side workstation, whose rotating magnet induces the particles to spin within the bloodstream. This activity creates flow towards the clot in otherwise stagnant vessels, bringing medications to the clot site 40 times faster than normal. The company has filed an early feasibility investigational device exemption (IDE) with the FDA, and anticipates beginning clinical trials to evaluate the device for stroke treatment later this year.
What's At Stake
Time is the enemy when treating many medical conditions, but nowhere is it a more threatening, disabling, and deadly adversary than in cases of stroke.
 Nearly 90 percent of all stroke cases are ischemic, caused by a blood clot that either originates in (thrombosis) or migrates to (embolism) a cerebral vascular artery that feeds the brain, effectively cutting off its blood supply.1 When deprived of oxygen-rich blood, brain tissues start to die within moments. In fact, it is estimated that some 1.9 million neurons, 14 billion synapses, and 7.5 miles of myelinated fibers are lost during every minute a stroke is left untreated, and each hour without treatment causes the brain to age the equivalent of 3.6 years. Hence the popular saying in stroke-treatment circles that “time is brain.”2
Nearly 90 percent of all stroke cases are ischemic, caused by a blood clot that either originates in (thrombosis) or migrates to (embolism) a cerebral vascular artery that feeds the brain, effectively cutting off its blood supply.1 When deprived of oxygen-rich blood, brain tissues start to die within moments. In fact, it is estimated that some 1.9 million neurons, 14 billion synapses, and 7.5 miles of myelinated fibers are lost during every minute a stroke is left untreated, and each hour without treatment causes the brain to age the equivalent of 3.6 years. Hence the popular saying in stroke-treatment circles that “time is brain.”2
Thus, the goal of ischemic stroke treatment is to open the blocked artery that supplies the brain, restoring oxygen supply as quickly as possible — but that’s often easier said than done. First, stroke symptoms need to be identified, and the patient needs to be rushed to the hospital. Next, a physician must rule out that the cause of stroke isn’t bleeding (hemorrhagic). Finally, the appropriate treatment has to be administered. All while the clock is ticking and the brain literally is dying.
The dominant treatment is intravenous delivery of tissue plasminogen activator (tPA), which dissolves the clot and restores blood flow to the brain. However, these clot-busting drugs must be delivered within 3 to 4 hours of an acute stroke, and even then are only marginally effective. If you took 100 patients with an ischemic stroke and provided only supportive care (oxygen, fluids, etc.), 26 out of 100 would be independent (not require assistance) in their daily activities. By adding tPA treatment, the number rises to 39, delivering benefit to 13 more patients per 100.3
However, over 60 percent of stroke patients, even with the best current treatments, fail to regain functional independence. “Better, faster methods for getting these blood vessels open are desperately needed,” says Colin Derdeyn, M.D., director of the Stroke and Cerebrovascular Center at Washington University and Barnes-Jewish Hospital, chair of the American Heart Association / American Stroke Association Stroke Council, and chair of the Pulse Therapeutics Scientific Advisory Board.
According to Sean Morris, CEO of Pulse Therapeutics, the intravenous delivery of tPA must overcome many barriers in order to get to the clot to do its job. One reason tPA isn’t more effective is its relatively short half-life. “About 10 percent of tPA therapy is delivered in an initial bolus, and then the rest is dripped in over the course of about 60 minutes,” he says. “Once the drug enters the blood pool, it is estimated to have about 5 to 10 minutes before the liver rips it apart and basically renders it useless.”
The other reason has to do with fluid dynamics. “Fluid always follows the path of least resistance,” Morris explains. “Since tPA is given in fluid form, intravenously, it naturally travels to arteries that are open, because they offer less resistance than in the blocked arteries you’re actually trying to reach. The tPA goes everywhere you don’t want it to go, and not where you really need it to go.” Blocked arteries are a lot like crimped garden hoses — nothing moves in them.
Due to poor delivery, given the suboptimal fluid dynamics, investigators have attempted several new methods for delivering tPA and other mechanical therapies directly to clot sites. For example, one approach involved inserting a catheter into the groin, threading it up through the artery, and dispensing tPA through it directly to the clot. This technique, termed catheter-directed thrombolysis, dramatically improved recanalization rates in blocked arteries. Unfortunately, this catheter-based tPA delivery method did not result in significant neurological improvement for patients. One major reason is because any gains in recanalization and reperfusion were offset by losses in time: time spent moving the patient to a cath lab, getting them catheterized, and administering the therapy.
The stroke community is very excited about another new technique that involves inserting catheters through the groin and directly to the clot. Called intra-arterial thrombectomy, this approach uses mechanical means to grab the clot and pull it out, and several recent trials have shown dramatic benefit for patients with occlusion of the large vessels at the base of the brain. These positive studies emphasize the potential of technologies that can open vessels faster and more effectively than tPA alone. On the other hand, the large majority of tPA-eligible patients have clots in smaller vessels, and thus are not eligible for the new thrombectomy devices.
Using Magnets To Solve tPA Delivery Challenges
Pulse Therapeutics’ MED System addresses both the fluid dynamics and time issues that have plagued tPA treatment.
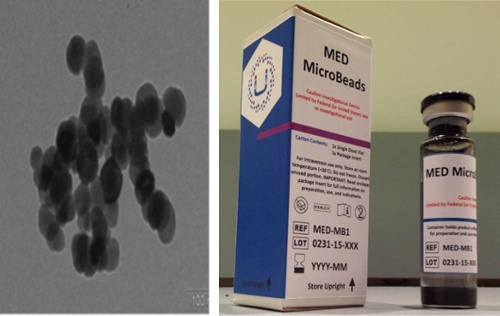
The system consists of two main components. The first is proprietary iron oxide particles, which the company has dubbed MicroBeads. These particles, which are one-fifth the size of a red blood cell, are infused into the bloodstream through a vein, after tPA therapy is initiated. Quickly, the MicroBeads disperse throughout the bloodstream.
 The second element of the system is the MED Workstation. This touchscreen-operated device, which is positioned near the patient’s head (without actually touching it), incorporates a softball-sized magnet that spins at 180 rotations per minute (RPMs). The force of the magnet draws nearby MicroBeads to the clot, causing them to tumble head-over-tail and creating flow in the previously stagnant artery. As a result, tPA also is pulled into the clot site.
The second element of the system is the MED Workstation. This touchscreen-operated device, which is positioned near the patient’s head (without actually touching it), incorporates a softball-sized magnet that spins at 180 rotations per minute (RPMs). The force of the magnet draws nearby MicroBeads to the clot, causing them to tumble head-over-tail and creating flow in the previously stagnant artery. As a result, tPA also is pulled into the clot site.
The system doesn’t just draw tPA to the blockage. Secondary mechanisms of action continually wash away the debris, bring in fresh tPA, and mix the tPA with plasminogen — the protein secreted by the liver that works in conjunction with tPA to break down clots. “It’s basically like keeping oxygen on the fire,” says Morris, “constantly mixing in more tPA before the drug becomes useless.”
Once the blocked vessel is opened (revascularized), the MicroBeads pass through the body’s exchange mechanisms and then are absorbed and excreted in the normal manner.
Other researcher groups have experimented with using magnets to move iron particles through the bloodstream, but what sets Pulse Therapeutics’ system apart is the patented rotating magnet, which used both force (pull) and rotation (gradient). This system works in tandem to move particles in a preferential direction.
“Others have tried to use a stagnant magnet — one big block of magnet,” Morris says. “The only way to make these systems slightly more effective is to make the magnet stronger, and the only way to do that is to make the magnet bigger. Eventually, the magnet becomes so big it’s impractical, and the results would still be minimal.”
Pulse Therapeutics founder and CTO Francis “Duke” Creighton, Ph.D., has a long history working with magnets and medicine: His thesis focused in large part on medical device applications for magnets and robotics, and he previously worked with magnets to bend metal-tipped catheters for various heart procedures.
Morris tells the story of Creighton’s eureka moment soon after establishing Pulse Therapeutics: “His proof-of-concept at the time was a magnet with a wooden hand crank, and he was testing to see if the mechanism could actually flip the magnet over and over again. Every time he turned it, he heard a clicking noise. There were little pieces of iron in the drawer in front of his lab, and they were flipping around inside as the magnet turned.”
“At that point he realized that instead of a piece of metal being stuck to some tissue in the body where it can never move, you could make the metal climb up or down or sideways or forward with a rotating magnet. And if you spin the magnet really fast, it is possible to really excite the iron particles and get them to move to areas in very new and exciting ways for many applications. Spinning a tire in the air does nothing, but if you put it on the ground, it takes off. That’s the concept,” he explains.
The results of this novel approach have been impressive in animal and early human testing. In 2013, Pulse Therapeutics conducted a pilot study with seven stroke patients in Australia, and the MED system successfully delivered tPA to the clot site in study patients at a rate much faster than normal diffusion. The results were presented at the International Stroke Conference in February 2015.
A Careful Regulatory Strategy
As is typically the case with pilot studies, Pulse Therapeutics’ 2013 research shed light on some aspects of the system that could be improved. The company has spent the last two years addressing those issues and gearing up for increased interaction with the FDA.
“We have had numerous meetings with FDA,” Morris says. “They look at the MED System as a very promising technology that may help to addresses one of the biggest global unmet needs.”
Pulse Therapeutics is working closely with the FDA in the early feasibility study program. The program allows companies to assess the safety and function of their devices with a limited number of patients, and to make timely design and clinical protocol modifications during the study. The goal is to streamline the clinical trials and commercialization process, accelerating U.S. patient access to promising new devices. Morris hopes to initiate human clinical studies later this year.
Along the development path, Pulse Therapeutics has taken great pains to avoid having its system disrupt the flow of normal stroke care. “We don’t want to do anything to interrupt the gold standard, so our process involves standard tPA administration, no matter what. Only after tPA is infused are our MicroBeads introduced,” Morris adds.
The Case For Payment
Each year, approximately 15 million people worldwide suffer a stroke. The condition strikes nearly 800,000 Americans per year, and it is fifth-leading cause of death and one of the top causes of disability in the United States.5
The death rate associated with stroke actually is falling, which is both a good and a bad thing. On one hand, it means that patients are getting to ERs much more quickly, getting treatment, and surviving stroke episodes. On the flip side, it means that the rate of stroke-related disability is on the rise, creating an increasing population of patients who are living out their lives incapacitated and dependent on long-term care. The economic burden of stroke on the U.S. healthcare system exceeded $80 billion in 2012, and that number is estimated to double by 2025.6
The company has begun doing research with private payers, exploring the different procedure codes that might be applicable and thinking through how to conduct clinical trials that will pass the test of evidence-based medicine. And Morris plans to initiate talks with the Centers for Medicare & Medicaid Services (CMS) after the company’s IDE is in place.
“We believe we have the potential to dramatically impact healthcare economics as it pertains to stroke,” he says. “To the extent we can provide outcomes benefits to stroke patients and keep more alive and less disabled, it will have a huge impact on costs.”
Ramping Up Funding
Over the last five years, Pulse Therapeutics has raised a rather modest $9 million in funding, much of it Morris attributes to “really strong internal investors” including FTL Capital, Cultivation Capital, and many interested high net worth investors.
“We are really efficient,” Morris says. “We have a team of six people, and we augment them with high-caliber consultants. This is what you get in our area of the country — a smart and lean team that can do much more with less capital. Not every great innovation comes from Silicon Valley or Cambridge.”
However, the company is redoubling its funding efforts, and is in the process of raising additional capital. Most of the funds will go toward supporting clinical trials, and the rest is earmarked for continued R&D and moving towards regulatory approvals.
The National Institutes of Health (NIH) also has lent its financial support, awarding Phase I and Phase II fast-track Small Business Innovation Research (SBIR) grants that will help Pulse Therapeutics expand its technology platform to new applications, including the treatment of blood clots that cause heart attacks.
“We believe that we have a valuable platform that can be useful in many ways and in many parts of the body,” Morris adds. “That said, we are focused on making the first application work.”
Challenges Ahead
For the next several years, Morris and his team will devote their full attention to studies and, assuming everything goes as expected, a pivotal trial for FDA approval. “A company can get into trouble when it starts to think about revenues,” he explains. “We are really focused on science and clinical data. What stroke needs is good, strong clinical data. If it has that, the revenues will follow.”
Even with such single-minded focus, the path to market will not be easy. Stroke is a challenging therapeutic area, primarily because of patient variability. Patients often are older, which means they can bring a wide range of other conditions to the table.
“Many companies have failed to bring stroke treatments to market, even with really strong animal, Phase 1, and Phase 2 data,” says Morris. “Once you introduce heterogeneity into a study — expand it to the masses — then you start to see how tough stroke is.”
Despite the long odds, Morris is confident that Pulse Therapeutics’ MED System will succeed where others have failed.
“We are not relying on some molecule to change the biological pathway. We are not another thrombectomy catheter that is limited by the number of doctors who know how to use it effectively and only when the circumstances are optimal, and by the fact that their instruments can only reach so far,” he says.
“What I like about our technology is that it's simple. We are merely parting the waters that exist to get the drug to where it needs to go,” Morris adds. “And there is a potential for us to go much, much deeper into these smaller vessels that other technologies could never reach. We merely rely on simple physics — it’s just magnetic pull and rotation.”
References:
- http://www.strokeassociation.org/STROKEORG/AboutStroke/TypesofStroke/IschemicClots/Ischemic-Strokes-Clots_UCM_310939_Article.jsp
- http://stroke.ahajournals.org/content/37/1/263.long
- http://www.ncbi.nlm.nih.gov/pubmed/7477192
- http://www.fda.gov/Training/CDRHLearn/ucm372150.htm
- http://www.cdc.gov/stroke/
- http://www.heart.org/idc/groups/heart-public/@wcm/@fda/documents/downloadable/ucm_462697.pdf
