Could Carbon Nanotube Fibers Replace Metal Electrodes In Deep Brain Stimulation?
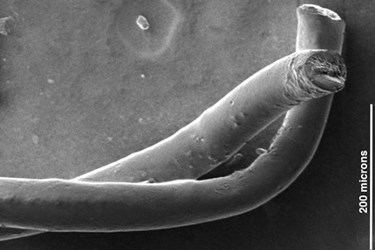
Engineers have constructed fibers from carbon nanotubes that can provide two-way communication with the brain. Researchers believe that the fibers could eventually replace metal electrodes traditionally used in deep brain stimulation (DBS), a treatment for Parkinson’s disease symptoms and other neurological disorders.
To create the fibers, a team of scientists from Rice University bundled millions of nanotubes in a process called wet spinning. The resulting thread is approximately one quarter the width of a human hair, according to a Rice press release. Originally intended for aerospace applications due to their strength and conductivity, the fibers are also very soft, a characteristic the researchers believe make them conducive to biomedical applications.
According to Matteo Pasquali, a Rice chemist and chemical engineer and leader of the research team, the softness was an unexpected bonus that makes the fibers “ideal for interfacing with the electrical function of the human body. They are really soft, much like a thread of silk.”
Pasquali took his initial findings to Caleb Kemere, a Rice assistant professor and an expert in animal models of Parkinson’s disease, who was eager to test the fibers in DBS therapy.
Their collaborative study, the results of which were published in ACS Nano, experimented in rat models by insulating the fibers with a three-micron layer of polymer and strategically placing the exposed tips using a brain atlas. Their experiments showed that the carbon nanotube fiber electrodes stimulated the brain as effectively as platinum electrodes but at a fraction of the size.
Furthermore, researchers found that the fibers provided a more favorable electrical connection than traditional metal electrodes and caused very little inflammation. This would allow electrical connections to remain strong over longer periods of time without interference from the immune system.
Flavia Vitale, lead author of the study, explained in the press release that traditional DBS first uses a probe to listen to neurons and determine placement of the metal electrode. Because the carbon nanotube fibers can both listen and stimulate, the placement process becomes much simpler.
The fibers can also respond to brain activity and adjust stimulation as needed in a self-regulating system, a feature that metal electrodes cannot provide.
Kemere said that eventually the team would like to develop a closed-loop system that can respond and adapt to the brain’s activity providing the maximum level of tailor-made treatment, which has been his goal since arriving at Rice University in 2012.
Last year, Rice University published a press release detailing Kemere’s plans to “reboot DBS” to make it even more effective for patients with neurological disorders.
“Though DBS is remarkably effective today, it provides only minimal therapeutic benefit for perhaps a third of Parkinson’s patients,” said Kemere in the 2014 press release. “It’s possible that a dynamic DBS could substantially increase effectiveness for these users.”
Image credit: Pasquali Lab, Rice University
