Dissolvable Circuitry For Medical Devices Developed From Egg Whites
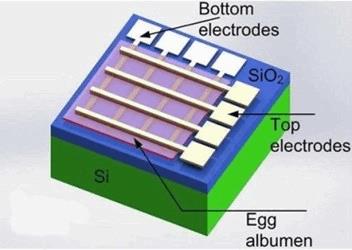
Using egg whites, magnesium, and tungsten, university scientists have developed a component of electrical circuitry that could be used in dissolvable implants or advanced drug delivery. Their lab tests showed that the circuits were as stable and reliable as non-degradable circuits and could be dissolved in water within 72 hours.
Traditional implants made of non-degradable materials typically remain in place even after the body has healed and the device has outlived its usefulness. Long-term implants can pose a risk of infection or other complications years after the initial procedure. In order to improve a medical device’s safety profile, researchers are working to make closed-loop devices from biocompatible materials that dissolve into the bloodstream with minimal side effects.
Scientists from Zhejiang University in China, in collaboration with Cambridge University in the U.K., have developed a memristor — a component of an electrical circuit that regulates current — by embedding magnesium and tungsten electrodes onto an ultra-thin albumen (egg white) film. In a study published in the Applied Materials & Interfaces, researchers demonstrated that their memristor could work for 3 months in dry settings and be dissolved in water in 3 days.
“The magnesium and tungsten electrodes and albumen film all can be dissolved in water within 72 hours, showing their transient characteristics,” said researchers.
In addition to its dissolvable properties, recent research has shown that albumen shows significant anti-microbial characteristics, a significant concern for medical implants. Scientists at the University of Georgia published a study in the Journal of Applied Polymer Science last year that showed that bioplastics made from albumen showed no bacterial growth after 24 hours of observation.
The Zhejiang/Cambridge team, led by Jikui Luo and Xiaozhi Wang, believe their transient electronics could prove useful in localized drug delivery or pollution monitoring, according to a press release.
“This work demonstrate a new way to fabricate biocompatible and dissolvable electronic devices by using cheap, abundant, and 100-percent natural materials for forthcoming bioelectronics era as well as for environmental sensors when the internet of things takes off,” stated the scientists in the study.
Research has shown that incorporating “smart” components into medication can improve its performance and efficiency. Christopher Bettinger, a biomedical engineer at Carnegie Mellon, explained that the inefficiency of traditional medications requires patients to consume more pills. If “smart components” could better direct the pills, patients ostensibly could consume fewer.
The FDA accepted the first application for a digital medication in September — a collaboration between Otsuka Pharmaceutical’s Ambilify and Proteus Digital Health’s ingestible sensor. The product uses sensors to monitor drug adherence and performance.
The FDA recently decided not to approve the digital version of Ambilify and has requested further data. A spokesman from Otsuka said in a statement that the companies are working closely with the FDA to resolve its questions.
