Electronic Implant Could Ease Hypertension
By Joel Lindsey
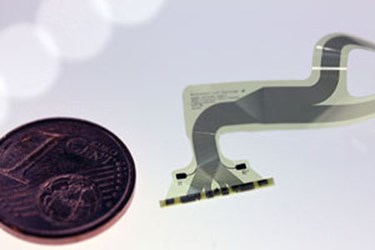
Researchers at the University of Freiburg in Germany have developed an implantable electronic device they say could use electrical pulses to help lower high blood pressure, especially in patients with hypertension.
Reports indicate that up to 35 percent of those with hypertension cannot be fully or effectively treated with drugs alone. The inability of drugs to treat all patients with hypertension is precisely what the new device is attempting to address, according to a press release published by the University of Freiburg.
“An implantable device would allow reducing the blood pressure in these patients, either alone or in combination with the already applied medication,” Dennis Plachta, a microsystems engineer at the University of Freiburg who is involved with the research project, said in an article published recently by MIT Technology Review. “It offers a second chance not available yet, and it can run in a tandem solution to a pharmaceutical treatment.”
As a solution, the research team created a tiny electrode-lined cuff, 20 mm long, that has been designed to wrap around a specific section of the vagal nerve, which is located in the neck and is responsible for delivering information between the brain and other important bodily organs — most notably the heart. The cuff would then be connected to an encapsulated small pulse generator, which would also be implanted beneath a patient’s skin. When implanted, the device’s electrodes could deliver pulses of electricity to the vagal nerve in order to stimulate blood pressure changes.
In early tests, researchers implanted the device in five adult rats and found that it helped reduce the rats’ blood pressure by as much as 40 percent, without causing any side effects, such as reduced heart or respiratory rates.
Findings from this study have been published recently in the Journal of Neural Engineering.
After completing these initial tests, researchers plan to focus on developing an implantable system that could one day be put into clinical tests and eventually gain approval for commercial markets. This process, however, could be a lengthy one, according to the University of Freiburg.
“As such a device is classed as an active implant that must fulfill the highest level of safety standards according to medical product laws, [researchers] do not expect to produce a licensed product for at least ten years,” according to the University press release.
This device is part of a growing field of research focused on using electrical signals to treat medical conditions by stimulating changes and responses in bodily functions. Med Device Online, for example, recently reported on the development of an implantable microchip that uses electrical pulses to restore movement in the muscles of paralyzed patients.
Image Credit: IMTEK
