FDA Approves Non-Surgical Balloon Device To Treat Obesity
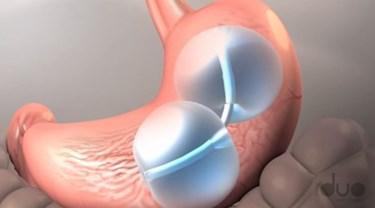
The U.S. Food and Drug Administration (FDA) on Tuesday approved the first intergastric balloon device for obesity treatment available in the United States. The ReShape Integrated Dual Balloon System is minimally invasive, reversible, and appropriate for patients with a body mass index (BMI) between 30 and 40 kg/m2 who do not qualify for bariatric surgery. However, while early studies look promising, experts urge caution until there is data to support the effectiveness of the device long-term.
The device works by occupying space within the stomach, which makes the patient feel full without changing the stomach’s anatomy. Using an endoscopic procedure, a clinician inserts the balloons into the stomach and, once they are in place, inflates the balloons with a sterile saline solution. According to an FDA press release, the device may trigger other mechanisms that are not yet fully understood.
The FDA has approved the device for patients having a BMI range between 30 and 40 and suffering from one or more conditions related to their obesity, such as high blood pressure, high cholesterol, or diabetes. The FDA indicated that the device should be used alongside a strict diet and exercise regimen, and only when that regimen has failed on its own.
According to the American Society for Metabolic and Bariatric Surgery (ASMBS), individuals with a body mass index BMI greater than 30 have a 50 to 100 percent increased risk of premature death compared to healthy weight individuals, as well as an increased risk of developing more than 30 obesity-related diseases and conditions including Type 2 diabetes, heart disease, and certain cancers.
“Many Americans who face the risk and consequences of obesity have struggled with a lack of effective weight loss options when their BMI is in the 30-40 range,” said John Morton, president of the American Society for Metabolic and Bariatric Surgery. “Options like ReShape address a significant gap that exists between diet and exercise, weight-loss medications and surgery, offering a minimally invasive, reversible option which we hope will promote long-term healthy habits.”
According to the announcement released by ReShape Medical, other balloon systems have been approved elsewhere in the world, but this is the first system approved for use in the United States.
“We looked closely at previous balloons when designing our dual balloon, and we feel confident that our unique next-generation design will help to deliver sustained results and significant life changes for patients,” said Rick Thompson, CEO of ReShape Medical.
A clinical study with 326 obese patients showed that the 187 patients with the device lost an average of 14.3 pounds in 6 months, twice the amount of weight lost by the control group. Six months after the device was removed, patients kept 9.9 pounds off.
Mitchell Roslin, chief of obesity surgery for Lenox Hill Hospital in New York, told US News and World Report (USNWR) that the procedure was unlikely to be an alternative for bariatric surgery or become part of the medical algorithm for treating obesity, and it will not be covered by any insurance plan for quite some time. USNWR noted that comparable systems cost $6,200 in Europe.
“It’s going to be effective for short-term weight loss,” said Roslin, “but there is still an overwhelming question whether these devices provide any long-term benefit for obesity.”
Roslin also noted that the procedure was not without side-effects, which include nausea, abdominal pain, and 35 percent of clinical trial participants developed gastric ulcers.
To mitigate some risk, the device’s saline is mixed with blue dye that is emitted through the patient’s urine if the device ruptures. In these cases, developers say that the device can be easily removed.
According to The Centers for Disease Control and Prevention (CDC), more than 72 million Americans have obesity and, according to ASMBS, about 24 million have severe obesity.
