FDA Goes 3-D
By Steven K. Pollack, Ph.D., and James Coburn, M.S., FDA Office of Science and Engineering Laboratories (OSEL)
A hospital in Michigan implants a 3-D printed medical device into a 3-month-old boy with a rare bronchial condition and saves a young life.
A man has 75 percent of his skull replaced with a 3-D printed implant.
3-D printing—the process of making a three-dimensional solid object of virtually any shape from a digital model—is making headlines these days, and the technology, once considered the wave of the future, is rapidly becoming part of the present.
It’s spurring innovation in manufacturing, dramatically reducing the time required to design new products and allowing designs to be built that were not possible before.

Dr. Steven Pollack (left) holds a 3D-printed RoboHand, a prosthetic for children with amnionic banding syndrome, an illness that can prevent fingers from developing in children. Research engineer James Coburn (right) uses the 3-D printer (background) in his work in the FDA lab.
Here at FDA, we’re using it to expand our research efforts and expand our capabilities to review innovative medical products. In fact, 3-D printing is fast becoming a focus in our practice of regulatory science—that is, the science of developing new tools, standards and approaches to assess the safety, effectiveness, quality and performance of FDA-regulated products.
With 3-D printing, the conversion from a virtual computer model to a physical object can occur almost in real time. The printer translates virtual models into digital cross-sections for use as a blueprint for printing, laying down successive layers in different shapes.
Two laboratories in the FDA’s Office of Science and Engineering Laboratories (OSEL) are investigating how the technology may affect the manufacturing of medical devices in the future.
At our Functional Performance and Device Use Laboratory we’ve developed and adapted computer-modeling methods to help us determine the effect of design changes on the safety and performance of devices when used in different patient populations. The 3-D technology enables us to tweak the design in ways large and small, and to see precisely how those tweaks will change both fit and functionality. In an era of increasingly personalized medicine, which involves the development of treatments that are tailored to an individual patient or a group that shares certain characteristics, including anatomical features, it helps us to fine-tune our evaluation of patient-fitted products.
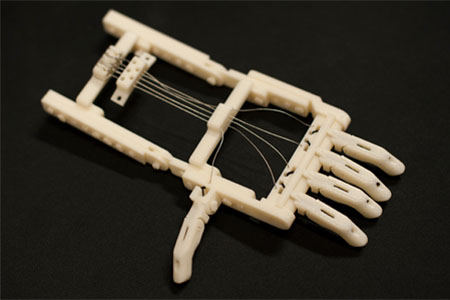
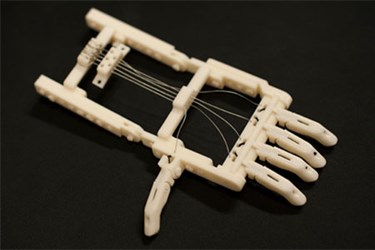
The Snap-Together RoboHand prosthetic was invented by South African carpenter Richard van As and made available for free on the Internet. Before printing, the hand can be individually sized, and all connecting pieces are also printed. The device can now be printed for less than $100.
At our Laboratory for Solid Mechanics we’re investigating how different printing techniques and processes affect the strength and durability of the materials used in medical devices. What we’re discovering will be valuable to our reviews of devices down the road; it will help us to develop standards and set parameters for scale, materials, and other critical aspects that contribute to product safety and innovation.
In August 2012, President Obama launched the National Additive Manufacturing Innovation Institute (NAMII), a national effort bringing together industry, universities and the federal government to provide innovation infrastructure to support new technologies and products created with additive manufacturing, the formal term for 3-D printing.
FDA has a long history of researching and regulating innovative technological practices. Regulators regularly review some of the newest technologies coming onto the market and, through our research, FDA has first-hand knowledge of these advanced techniques so we can evaluate advanced technology at an early stage—a crucial step in facilitating innovation and protecting the public health. We will continue to facilitate device innovation and keep on the cutting edge of technology and regulatory science to help ensure that the products we regulate are safe and effective.
To see more photos of how FDA is using 3-D printing technology, visit our Flickr photostream.
About The Authors
Steven K. Pollack, Ph.D. is Director of FDA’s Office of Science and Engineering Laboratories (OSEL) at FDA’s Center for Devices and Radiological Health. James Coburn, M.S. is a Research Engineer in OSEL.
This blog post originally appeared on FDA Voice, the FDA's official blog brought to you from FDA's senior leadership and staff stationed at home and abroad — sharing news, background, announcements and other information about the work done at the FDA on behalf of the American public.
