Harvard, UMass Collaboration Creates Drug-Device Combination For Stroke Treatment
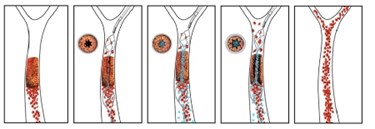
A newly developed drug-device combination can burrow through a stroke-causing clot while releasing anti-coagulant medication. The injectable nanotherapeutic targets blocked vessels and works in tandem with a temporary endovascular bypass (TEB) procedure to remove clots with fewer side effects than a stent-retriever thrombectomy (SRT).
Researchers from Harvard University’s Wyss Institute (Wyss) collaborated with the University of Massachusetts’ New England Center for Stroke Research (NECSTR) to coat biodegradable nanoparticles with a tissue plasminogen activator (tPA) — the current gold standard in ischemic stroke treatments. The scientists stated in a press release that blood flow naturally increases at the blockage location, and this mechanical force releases the coated nanoparticles, which bind to and dissolve the clot.
Recently, researchers have been investigating the SRT procedure, which inserts a tube into the clot along with a closed stent. Once the stent is opened, surgeons use the stent to drag the clot out of the vessel. While studies have shown this procedure is more effective than pharmaceutical intervention alone, SRT still carries some risk, said Matthew Gounis, associate professor of radiology at the University of Massachusetts (UMass), who helped develop the procedure.
“Even with the retriever thrombectomy procedure, not all clots can be removed with a successful outcome,” said Gounis. “Clot fragments can be dislodged, which can lead to microclots and tissue damage downstream in the brain’s circulatory system, and physical dragging of the stent through the vessel can potentially be damaging as well.”
Donald Ingber, founding director at Wyss, and Ajay Wakhloo, professor of radiology at UMass, led a study — published in Stroke — describing a new approach using the drug-device combination during a TEB procedure to see if outcomes were more successful than with stent retriever thrombectomy. Their approach uses the stent to open the blockage, but allows the activated nanoparticles to finish the job of dissolving the clot.
Their study with large animals — whose clots were similar in size to clots found in humans — found that the approach achieved “high rates of recanalization” and reduced vascular trauma as compared with stent-retriever thrombectomy alone.
“What’s progressive about this approach is that the temporary opening of a tiny hole in the clot—using a stent device that is already commonly used clinically—results in a local rise of mechanical forces that activate the nanotherapeutic to deploy the clot-busting drug precisely where it can best do its job.”
A 2013 report released by Transparency Market Research estimates that the market for drug-device combinations is expected to reach $115.1 billion by 2019, and last July, the U.S. Senate introduced a bill that would streamline the regulatory process for combination products.
Michael Drues, president of Vascular Sciences, recently observed in an MDO guest column that “Approximately one third of all medical products under development today are combination products. This is clearly an area of rapid growth and great opportunity for medical device makers.”
