Heart Pump Manufacturer Issues Safety Warning In Wake Of Deaths
By Joel Lindsey
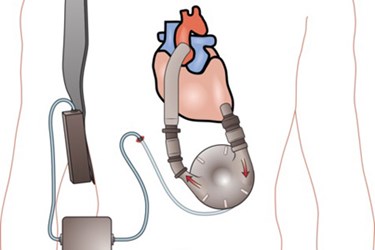
Heart pump manufacturer, Thoratec Corp., issued a safety warning last week after four patients died and five others were injured while using one of its products.
The warning focused on the company’s HeartMate II Left Ventricular Assist System (LVAS) pocket controller, and reported that the deaths and injuries were sustained when patients struggled switching from the main pump controller to the backup.
Thoratec’s HeartMate II is implanted inside a patient’s chest to help the left ventricle pump blood through the body. The pump is then attached to an external controller that monitors the pump’s performance, alerting patients to emergency power shortages or when the pump’s battery pack is running low.
In its safety warning, Thoratec claimed that eight of the nine incidents occurred when patients attempted to switch from using an older model of the HeartMate II controller to a newer one. Thoratec also said that two of those who died had attempted to switch controllers while alone and without contacting the hospital first, both of which are contrary to the device’s labeling.
“You have to disengage the pump to change the controller,” Don Middlebrook, Thoratec’s VP of corporate regulatory affairs and quality assurance, said in a news article published by The Wall Street Journal. “If you struggle with that, you can become dizzy or unconscious.”
Some healthcare professionals see these incidents as primarily a failure on the part of doctors and care providers to adequately educate their patients.
“It’s a new controller,” Dr. Liviu Klein, a cardiologist at the UCSF Heart and Vascular Center, said in an article published by SFGate. “The blame I don’t think falls on the company. It falls on the physicians and the caregivers that did not spend enough time on the patients, did not spend enough time making sure they get the appropriate training.”
According to an article published by Bloomberg Businessweek, 2,142 patients have been prescribed the new HeartMate II pocket controller since August 2012. Many of these prescriptions were given to patients who had previously been trained to use the older model.
Thoratec has not found any significant defects in the HeartMate II system and has not issued a recall on the device. The company also noted that HeartMate devices have been implanted in thousands of patients around the world, and that these implants have successfully helped increase survival rates while also decreasing complication rates.
A recall was issued, however, in 2012, when Thoratec discovered a defect that could have cut off blood flow from the pump and led to serious complications, according to SFGate. Similarly, a study published this past January in The New England Journal of Medicine found “an apparent increase in the rate of device thrombosis among patients who received the HeartMate II left ventricular assist device.”
Image Credit: “Ventricular Assist Device.” © Creative Commons Attribution-ShareAlike 3.0 Unported: http://creativecommons.org/licenses/by-sa/3.0/deed.en
