Hybrid Cochlear Implant Restores High Frequency Hearing
By Chuck Seegert, Ph.D.
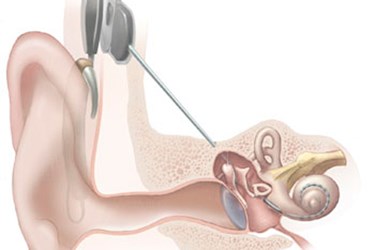
Severe loss of high-frequency hearing affects many people for a variety of reasons, often making it difficult for them to hear faint sounds, high-pitched noises, and even emergency vehicle sirens. To address this issue, a company in Australia has developed an implant designed to restore high-frequency hearing, while leaving the patient’s functional low frequency hearing intact.
Damage to the inner ear, or cochlea, can be caused by aging, loud noises, drugs like antibiotics, genetic anomalies, and other illnesses that may affect the inner ear. A common approach to dealing with this type of hearing loss is to use a hearing aid. Unfortunately, however, hearing aids are not always effective. Additionally, existing cochlear implants are better-suited for patients who suffer from total hearing loss that includes high and low frequencies.
“Many patients who have been frustrated with their hearing aid performance, but had too much low frequency hearing to qualify for a cochlear implant, may now qualify for the hybrid implant,” said Gail Murray, Ph.D., director of the Audiology and Cochlear Implant Center at University Hospitals (UH) Case Medical Center and associate professor of otolaryngology at Case Western Reserve University School of Medicine, in a recent press release.
The new implant, called the Nucleus Hybrid L24 Cochlear Implant System, received approval this past spring for use by the FDA. It has only been implanted at a handful of medical centers in the United States, including UH Case Medical Center in Cleveland, OH. The surgery was performed by Dr. Semaan, who is the associate director of otology, neurotology, and balance disorders at UH Case Medical Center and assistant professor of otolaryngology at Case Western Reserve University School of Medicine. The patient was a male in his sixties who had lost hearing in the high-frequency range.
The surgery only took about 2 hours to perform, but the implant won’t be tested until the patient has had about 6 to 8 weeks to recover, at which time the team hopes to see significant improvement for the patient.
“Hearing loss greatly impacts the quality of a patient’s life,” said Dr. Semaan in the university’s press release. “When compared to the benefits provided by conventional cochlear implantation, the hybrid implant device may enhance the sound richness and quality, music appreciation, and improve hearing in noise.”
The new L24 implant is manufactured by Cochlear Ltd., a company headquartered in New South Wales, Australia. The device is composed of a speech processor that converts sounds picked up by an external microphone into electrical impulses. Implanted electrodes transmit the electrical impulses to the cochlea, thus creating a sense of sound that the patient learns to associate with mid- and high-frequency sounds. A hearing aid portion similar to a conventional hearing aid is also inserted into the ear for amplifying low-frequency sounds.
Advances in cochlear implants continue as technologies like wireless recharging make implants that have no external components possible.
