Medtronic Makes Significant Strides Toward Artificial Pancreas
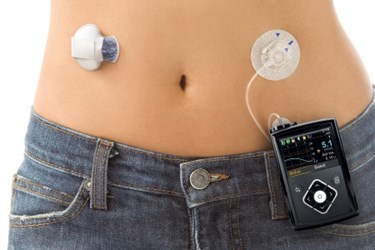
Medtronic’s new insulin pump, the MiniMed 640G, has been approved for use in Australia with other markets around the world expected to follow. The advanced technology mimics human pancreatic function, and Medtronic is calling the launch a breakthrough towards the development of an artificial pancreas.
The MiniMed 640G is equipped with the Bayer CONTOUR NEXT LINK 2.4 blood glucose meter that feeds readings into a Bolus Wizard calculator to reduce the risk of manual error, according to a recent press release from Medtronic. Additionally, Medtronic’s SmartGuard technology stabilizes blood glucose by starting and stopping the insulin pump as needed.
The device is completely portable, waterproof, and requires little input from the user, though patients can discreetly administer a bolus of insulin if required. Furthermore, the programmed limits are customizable, and alerts can be tailored to suit the individual patient’s needs.
Tim Jones, clinical professor at the University of Western Australia and head of the diabetes department at Princess Margaret Hospital in Perth Australia, calls the technology a “very valuable tool for achieving better glucose control,” according to the Medtronic press release.
Jones explains that because the system can detect both low and high glucose levels and can start and stop the insulin pump accordingly, both hypoglycemic and hyperglycemic attacks can be managed automatically.
“Managing hypoglycemia and rebound hyperglycemia after treatment is one of the biggest challenges in managing diabetes,” Jones added in the press release.
Alejandro Galindo, vice president and general manager of the Intensive Insulin Management business at Medtronic, remarks that the system was the result of many years of collaboration in the diabetes research community.
According to Galindo, this launch marks the company’s third milestone on the road towards developing an artificial pancreas.
In 2005, the Juvenile Diabetes Research Foundation (JDRF) launched the Artificial Pancreas Project (APP) and made significant investments in technological research.
A report from the American Diabetes Association estimated that since 2010, there have been over 40 clinical trials testing versions of an artificial pancreas.
Francis Doyle, department chair of chemical engineering at the University of California, Santa Barbara, told the American Diabetes Association, “there is no one single artificial pancreas,” and that there are many versions under development around the world.
According to the JDRF website, an estimated three million Americans are currently managing type 1 diabetes, and the number of diagnosed cases, especially among children, is rising. Type 1 diabetes accounts for $14.9B in U.S. healthcare costs each year.
If an artificial pancreas is developed and approved in the U.S., the JDRF estimates a total healthcare savings of $1B over the next 25 years.
While Australia is the only market currently approved for the MiniMed 640G, the system is under regulatory review in several other markets where it is expected to launch later this year.
Image Credit: Medtronic
