MR Imaging Plays Pivotal Role In Stem Cell Tracking
Intravenous iron can be used to safely and effectively label stem cell transplants for tracking with MR imaging in arthritic joints and other target tissues, new research shows.
Intravenous iron can be used to safely and effectively label stem cell transplants for tracking with MR imaging in arthritic joints and other target tissues, according to a recent Radiology study.
Bone marrow-derived mesenchymal stem cells (MSCs) have great potential in tissue regeneration and cell-based therapy, according to Heike E. Daldrup-Link, M.D., Ph.D., co-author of the study that appeared in the October issue of Radiology. Once transplanted from donor into recipient, MSCs can help repair damaged joints by giving rise to connective tissue, bone and cartilage, but the stem cells should be tracked to confirm the procedure’s success, Dr. Daldrup-Link said.
“The most common problem is that stem cells, when transplanted, die and disappear from the transplant site,” said Dr. Daldrup-Link, an associate professor in the Department of Radiology and a member of the Molecular Imaging Program at Stanford University School of Medicine. “Alternatively, they stay in the correct site but don’t differentiate into cartilage.”
Current labeling methods involve removing stem cells from a donor and then placing the cells in a culture dish with an iron oxide solution. The iron oxide-labeled cells are then transplanted into the recipient patient. Such ex-vivo labeling requires handling of the stem cells between harvest and transplantation, introducing the possibility of contamination.
“Orthopedic surgeons want labeled stem cells, but not the ones that are manipulated between the bone marrow harvest and transplantation,” Dr. Daldrup-Link said.
The Stanford researchers theorized that they could more effectively label the stem cells through an in-vivo approach.
“Our solution was to give an iron supplement to the stem cell donor intravenously before harvesting,” Dr. Daldrup-Link said. “The donor cells, including bone marrow stem cells, pick up the iron oxides, and these stem cells can then be tracked with MR imaging.”
In-Vivo Approach Shows Promise

Prior to testing in humans, researchers conducted an animal study, injecting rats with ferumoxytol, an FDA-approved iron supplement for treating patients with iron deficiency anemia, 48 hours prior to extraction of the stem cells from bone marrow. They then compared the ferumoxytol uptake by the stem cells with results from traditional ex-vivo-labeling procedures.
“To our surprise, we found that the stem cells take up significantly more iron with the intravenous labeling procedure than they do if we label them ex vivo,” Dr. Daldrup-Link said.
After transplanting the labeled stem cells into cartilage defects in the knees of seven rats, researchers performed MR imaging to track the cells for up to four weeks. Microscopic examination confirmed the presence of iron in the labeled transplants and showed evidence that repair was underway in the damaged joints.
In-vivo labeling not only eliminates the risk of contamination from ex-vivo labeling procedures, but also provides more immediate feedback on the status of the cells.
“The current approach to determine successful engraftment requires long-term follow up that imaging studies weeks or months after cell transplantion, even though the stem cells may die relatively soon after the procedure,” Dr. Daldrup-Link said. “With this method, we could show more quickly if cells disappear from the transplant site or if they proliferate too much after the transplant.”
The Stanford researchers are completing more examinations and plan to study the technique in rabbits before patient trials begin. Since the intravenous iron solution is widely used on anemic patients, Dr. Daldrup-Link believes that patient trials are just around the corner.
MR Imaging Holds Great Potential in Cell Tracking
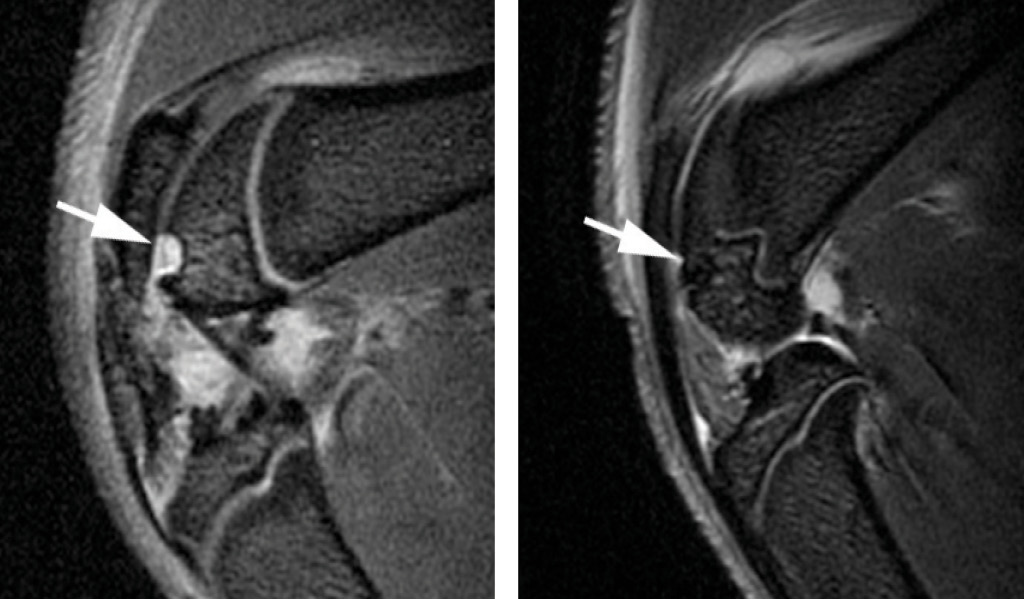
The findings point to the ultimate clinical role of MR imaging for cell tracking: monitoring the accuracy of cell injection in real time with MR-compatible catheters, according to Jeff W.M. Bulte, Ph.D., director of cellular imaging at Johns Hopkins University’s Institute for Cell Engineering in Baltimore, Md., and one of the world’s leading authorities on stem cell transplantation and tracking.
Dr. Bulte, who authored a Radiology editorial accompanying the research, said that the ability to track MR-labeled MSCs safely and effectively will have myriad clinical applications well beyond the treatment of damaged joints.
“These techniques are universal and could have many potentially useful applications, including heart repair and repair of brain neurons,” he said.
Noninvasive monitoring of the movement and accumulation of stem cells would enable clinicians to determine whether cell delivery has occurred in the appropriate location and whether transplanted cells have reached the appropriate location for each patient.
“We can’t take biopsies, so noninvasive imaging is the way to go in the future,” Dr. Bulte said. “It’s sort of like FedEx tracking your packages to make sure they reach their destination.”
Dr. Bulte cautioned that there are several limitations to the iron-oxide labeling approach. Not all donors yield sufficient numbers of MSCs and efforts to get a sufficient number may dilute the ferumoxytol label to uncertain cellular detection levels. In addition, the approach cannot distinguish live cells from dead cells and has difficulty differentiating between labeled MSCs and macrophages—the immune cells that engulf cellular debris and pathogens.
Labeling stem cells with fluorine represents a promising alternative that may overcome some of the limitations of the iron oxide method, Dr. Bulte said. Because fluorine resonates at a different frequency than hydrogen, the MR imaging coil can be tuned to its specific frequency. Since there is little to no fluorine in the body, the signal from the labeled fluorine can then be easily distinguished from any background noise and the quantity of the labeled cells can be determined with accuracy.
The labeled fluorine approach was first tested on a patient in April at the University of Pittsburgh Cancer Institute in Pittsburgh, and studies are ongoing.
Source: Radiological Society of North America
