Philips, Banyan Collaborate On Blood Test For Concussions
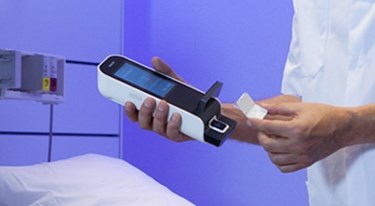
Royal Philips has announced a multi-year partnership with Banyan Biomarkers to co-develop a point-of-care diagnostic test for traumatic brain injury (TBI), otherwise known as concussion. The handheld device will leverage Banyan’s research in isolating blood-based biomarkers for TBI with Philips’ Minicare I-20 platform, and will be commercialized for use in emergency departments, which may help reduce healthcare costs associated with medical imaging.
Current standards for TBI diagnostics rely heavily on subjective neurological and cognitive examinations performed by emergency technicians and specialists, who test the patient for problems with vision, hearing, concentration, balance, and memory. According to the Mayo Clinic, physicians may order cranial computerized tomography (CT) scans or magnetic resonance imaging (MRI) if they suspect cranial bleeding.
In 2010, Banyan Biomarkers received a $26.3 million contract from the U.S. Department of Defense to develop diagnostic technology that could detect TBI with a blood test. Last year, the company was awarded $500,000 by the GE and NFL Head Health Challenge, a prize given to six finalists out of 400 applicants who presented research that investigated potential TBI biomarkers.
“The ability to detect neural injury is the first step in developing an objective test for TBI and ultimately make contact sports safer for all participants,” said Jackson Streeter, CEO of Banyan Biomarkers, in a press release.
Last November, the Banyan Biomarkers announced positive clinical trial results — published in the Journal of Neurotrauma — which indicated 100 percent of trial participants who tested positive for specific biomarkers in the Banyan test went on to have positive CT scans indicating TBI. A simple blood test, said researchers, may help reduce radiation risk and healthcare costs associated with medical imaging. Results from a larger study with 2,000 participants are expected later this year.
Banyan Biomarkers’ partnership with Royal Philips, for which financial details were not disclosed, will combine the biomarker research with the Minicare I-20 platform, handheld technology aimed at providing point-of-care blood tests that can provide results within minutes.
In a press release, Marcel van Kasteel, CEO of Philips’ handheld diagnostics unit, commented these devices would “play a critical role in improving patient outcomes and reducing healthcare costs. Our Minicare technology platform and partnerships are a firm foundation for our ambition to become a leader in this new growth market.”
Last March, Royal Philips announced a partnership with Janssen Pharmaceutical to develop hand-held blood tests to detect neuropsychiatric disorders.
The U.S. Centers for Disease Control and Prevention (CDC) reports that 2.5 million emergency department visits, hospitalizations, and deaths are associated with TBI, and represent 30 percent of injury deaths in the U.S per year.
Streeter stated in a press release that a point-of-care device for TBI diagnostics would directly impact millions of lives, saying, “This partnership with Philips to develop and commercialized a TBI blood test on the Minicare system is an important step forward in providing physicians and other healthcare providers information to evaluate patients who have suffered a concussion.”
