Second Sight's Bionic Eye Implanted In First U.S. Patients
By Joel Lindsey
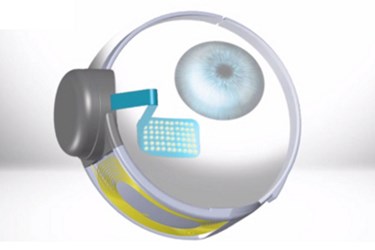
In January, surgeons at the University of Michigan completed the first two implants of Second Sight Medical Products' Argus II Retinal Prosthesis System in the United States.
Thiran Jayasundera, M.D., and David N. Zacks, M.D., Ph.D., performed the operations at the University of Michigan’s Kellogg Eye Center. The first was completed on January 16 and the second on January 22.
“We are pleased with both patients’ progress at this point, and we are hopeful and optimistic that the artificial retina will enable them to see objects, light and people standing before them,” Jayasundera said in an article published by the University of Michigan. “We believe the device will help them navigate a little better at home, be more independent, and have the pleasure of seeing things that the rest of us take for granted.”
Last month’s operations occurred nearly one year after the FDA cleared the Argus II prosthetic device for use in the U.S. The prosthetic, which was officially approved on February 14, 2013, is the first of its kind to receive the FDA’s sanction.
“The device, which includes a small video camera, transmitter mounted on a pair of eyeglasses, video processing unit and an implanted retinal prosthesis, replaces the function of degenerated cells in the retina and may improve a patient’s ability to perceive images and movement,” the FDA said in its news release regarding the approval. “The [video processing unit] transforms images from the video camera into electronic data that is wirelessly transmitted to the retinal prosthesis.”
Retinal prosthesis implants are designed to restore vision to those diagnosed with retinitis pigmentosa (RP), an inherited degenerative disease that causes gradual vision loss due to a decrease in the number of rods and cones in the retina.
In addition to Second Sight’s already-FDA approved Argus II, there is a growing body of retinal prosthetic devices in development. For example, IIP-Technologies GmbH is developing a device called the Learning Retinal Implant, a group of German developers is working on a wireless prosthetic called the EPI-RET-3, and German-based Retina Implant AG has also put a prosthetic device into trials.
While many researchers, developers, surgeons, and patients are optimistic about the potential of retinal implants to reverse the effects of RP, retinal implants do not yet promise fully restored vision.
“I understand that I will not have 20/20 vision and that I won’t be able to distinguish faces,” said 65-year-old Linda Schulte, the first person to receive retinal implants at UM last month. “But at least I will be able to know that my grandchildren are running across the yard or walking into my house. That would be a miracle to me.”
