First Implants In U.S. Trial Of St. Jude's Repositionable Transcatheter Aortic Valve System
By Joel Lindsey
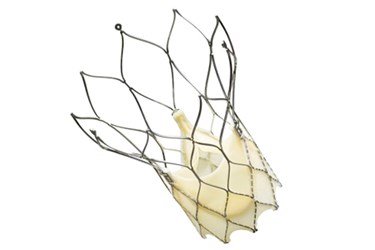
The first U.S. implants of St. Jude Medical’s new Portico Transcatheter Aortic Valve System have recently occurred as part of a new clinical trial of the device.
“As we continue to collect clinical evidence on the best way to treat patients identified as high or at extreme risk for the open-heart valve replacement procedure, the Portico valve represents a life-saving treatment option,” Gregory P. Fontana, cardiac surgeon and chairman of the department of cardiothoracic surgery at Lenox Hill Hospital in New York, said in a press release published recently on St. Jude’s website. “The valve and delivery system were designed to more safely treat heart failure symptoms in patients with stenotic valves.”
The current test of the Portico valve system is focusing on using the device to treat those with symptomatic severe aortic stenosis, a condition in which a patient’s aortic valve narrows to the point that it impedes the flow of blood out of the heart.
According to the press release, the Portico device is the first aortic heart valve “that can be completely resheathed (the process of bringing the valve back into the delivery catheter), repositioned at the implant site, or retrieved before being released from the delivery system.”
The device is delivered to a patient’s heart via catheter, typically through a transfemoral artery. Once it reaches the heart, surgeons can position the device and partially deploy the valve in order to test whether or not it has been placed correctly. After testing and if needed, the device can be fully resheathed and repositioned until the proper placement has been achieved, at which point surgeons can fully deploy the valve and remove the delivery system back through the artery.
“The ability to fully resheath and precisely reposition the Portico valve at the implant site prior to valve deployment helps achieve accurate placement, which may simplify the implant procedure and help minimize procedural risk for the patient,” said Raj Makkar, director of interventional cardiology at Cedars-Sinai Heart Institute in Los Angeles. Makkar and Fontana were both involved with the first round of Portico implants in the U.S.
By designing an aortic valve system that can be inserted into the aorta via catheter, positioned, tested, and repositioned before being fully deployed, St. Jude claims that its new device could make new modes of treatment possible for patients suffering from heart conditions who cannot receive open heart surgery.
“The Portico valve is an attractive option that will enable interventional cardiologists and cardiac surgeons who perform TAVR (transcatheter aortic valve replacement) procedures to treat patients who might not otherwise be eligible for surgery,” said Mark Carlson, chief medical officer and VP of global clinical affairs at St. Jude Medical.
The Portico device has already been approved for use in Europe, and the results of the current U.S. trial will be used to gain FDA clearance. The recently launched Portico clinical trial will treat patients at up to 40 U.S. sites and will gather data for review and analysis.
