Stryker Leverages Ultrasonic Energy For Stronger Soft Tissue Anchor
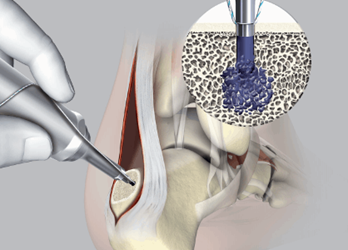
Stryker has introduced a soft tissue fixation system, called SonicAnchor, for use in extremity repair surgeries that reattach tendons to bone. The technology liquefies the tip of the polymer implant using ultrasonic energy and the material flows into cancellous bone cavities, where it solidifies for a more stable hold.
Many common injuries require the reattachment of tendon to bone, but research shows that these repairs are often mechanically inferior and result in reduced strength and load bearing, according to a study in Muscle, Ligaments, and Tendons Journal. Research also shows that reattachment to compact bone, rather than cancellous or spongier bone, usually results in more positive outcomes.
Stryker’s SonicAnchor was developed specifically for reattachment surgeries — such as Achilles tendon repair, hallux valgus reconstruction, carpal ligament reconstruction, and digital tendon transfer — where the tendon must be attached to cancellous bone. The 2.5 mm bioresorbable implant is first melted and then fused with the porous bone, according to a press release, which provides “a significant enhancement in dynamic load stability” over traditional devices when used without the presence of more compact bone material.
“This product’s long resorption time and excellent biocompatibility have made issues such as sterile abscess and foreign body reaction seen with other biodegradable products nonexistent in my significant experience with this material,” said Thomas Rocchio, a fellow of the American College of Foot and Ankle Surgeons, to Podiatry Today last year.
According to Stryker, SonicAnchor is less likely to break upon insertion and demonstrates a higher “pull-out strength and reliability.” Once inside the bone cavities, SonicAnchor solidifies within five seconds.
Stryker intends to present the technology in Toronto at the American Orthopaedic Food & Ankle Society (AOFAS) annual meeting later this month. Stryker also plans to unveil M.O.R.E — an educational tool that trains surgical staffs in the use of Stryker products — the Hoffmann Limb Reconstruction Frame, and a new Anchorage CP plating system.
In March, Stryker received FDA clearance for a titanium PL posterior lumbar cage that aids in spinal fusion and fixation for patients with degenerative disc disease.
Stryker has been on an acquisition spree this year, snapping up new technology in a variety of business segments, including oncology, neurology, and spine. Stryker CEO Kevin Lobo commented that Stryker is most interested in “tuck in” deals that expand the company’s existing business segments. The company’s largest acquisition was Physio-Control, a $1.3 billion, all-cash transaction to acquire a suite of emergency services devices.
