Sustainable Innovation: A Roadmap For Medical Device Manufacturers
By Jim Pomager, Executive Editor
Does the medical device industry have an innovation problem?
To the uninitiated, the notion probably seems ludicrous — after all, we’re talking about a sector designing astonishing technologies like bionic eyes, artificial pancreases, Tricorder-inspired diagnostic tools, and mind-controlled prosthetics. However, as anyone involved in product development knows, there’s a significant difference between invention and innovation. It’s one thing to devise a device that the world has never seen, and it’s another to persistently improve upon an existing design (yours or a competitor’s) until it’s tailor-fit to meet a real market need. And while both elements are critically important to the success of a technology, invention without innovation falls short of its potential — from both utilitarian and economic perspectives.
The trouble is, innovation management isn’t easy or straightforward. It requires a high level of analytical and operational rigor — as well as significant institutional support — to effectively drive and sustain innovation within an organization. And if you asked medtech product development folks to evaluate their companies’ current innovation management programs, most would tell you there’s a lot of room for improvement.
This was one of many interesting findings presented during a webinar I attended titled The State of Product Development in 2013 — Medical Device Manufacturers: A Need for Speed and a Roadmap to Sustainable Innovation, hosted by the Medical Devices Group on LinkedIn. During the presentation, Carrie Nauyalis of Planview (a portfolio and resource management company) and Maureen Carlson of Appleseed Partners (a marketing consulting firm) shared the results of a recent study exploring innovation at companies with complex product portfolios, including medical device makers.
The study, commissioned by Planview and conducted by Appleseed and OpenSky Research during the months of August and September 2013, sought to identify the biggest current challenges, risks, and trends in product portfolio management — and make recommendations for fast and sustainable innovation practices. The project involved qualitative telephone interviews and follow-up online surveys with more than 500 individuals involved in the product development process. Participants worked at companies ranging in size from 99 employees to more than 25,000 employees, and from $100 million in annual revenue to more than $1.1 billion. While the study involved respondents from around the world, most (85 percent) were based in North America or Europe. Over 90 medical device manufacturers participated.
Innovation Management Maturity: Where Do Medtech Firms Rank?
To better interpret trend information in this year’s survey (the fourth since 2009), Planview developed a concept they call “innovation management maturity.” Within the firm’s Innovation Management Maturity Model framework, respondents ranked their companies from Level 1 (low) through Level 5 (high) across three core areas of innovation management: People, processes, and tools. The people category covers things like assigning innovation responsibility to specific individuals (organizational structure) and establishing a center of excellence to manage innovation. Processes include using Stage-Gate or phase-gate models, from ideation to launch and through to sunset. And tools refers to any type of automated application or software that is integrated across an enterprise. Click on the image below for a more detailed breakdown of each category by innovation maturity level.
When asked to grade themselves across these vectors, device manufacturers exhibited a pretty sober self-evaluation. Nearly 80 percent of those that participated rated their organizational maturity level as average or below (Levels 1 through 3) in terms of people and tools, and almost 75 percent classified themselves the same way in terms of process. As for overall innovation maturity, more than 80 percent ranked themselves in Level 3 or below.
Nauyalis pointed out that not all device makers do — or should — aspire to Level 5. “You need to decide for yourself as a company, a brand, or a particular product line what your level of maturity needs to be,” she said. “Maybe you don’t need a center of excellence to manage the process, tools, strategies, and people behind the innovation and growth, but maybe you do. Only you can decide.” In fact, among the medtech companies surveyed, a combined 53 percent said they want to attain Level 4 or 5 maturity status, and 22 percent indicated their end goal is only Level 2 or 3. (As for the rest, 18 percent said they “don’t know” and another 8 percent — some of whom were likely among those who already rank themselves as Level 4 or 5 — said they have “no desire to advance”).
Common Pain Points And Risks
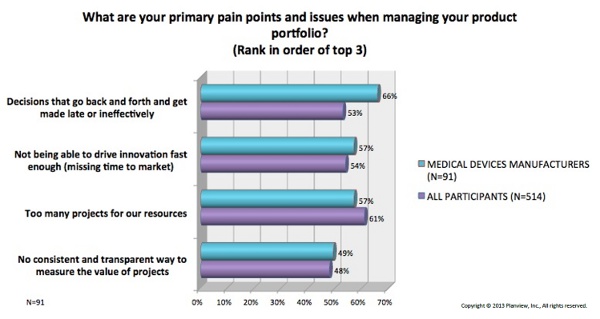
The study data revealed a shared set of challenges many device makers face in effectively managing their product portfolios, and trending data (over four surveys) shows that these pains are worsening. In order of precedence among medtech companies, the top four were:
- Decisions that go back and forth and get made late or inefficiently
- Inability to drive innovation fast enough (missing time to market)
- Too many projects for available resources
- No consistent and transparent way to measure the value of projects
The graph below shows percentages of participants who said each pain point applied to their organization, comparing medical device manufacturers with companies from other industries.
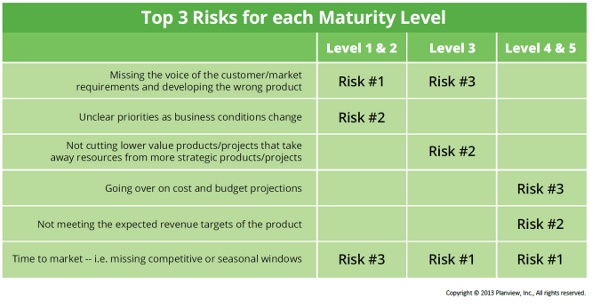
On the risk front in managing a product portfolio, medtech participants were most concerned with (in order of overall importance):
- Time to market (missing competitive or seasonal windows)
- Unclear priorities as business conditions change
- Failing to meet the expected revenue targets for the product
- Missing the voice of the customer and the market requirements, and thus developing the wrong product
It’s important to note that risks shift based on the current maturity level of the company. As you can see in the chart below, people at Level 1 and 2 companies (the least “mature”) are worried about developing the wrong product and having unclear priorities, two priorities that don’t even crack the top three for those at Level 3 and up, who are principally concerned about time to market and meeting revenue targets.
4 Missing Elements For Speeding Innovation
It’s clear from the top pain points and risks that speed is the primary goal of medtech companies when it comes to product portfolio management. So what’s holding (most of them) back? According to the study, there are four missing components to move innovative medical devices through the development pipeline more quickly. They are:
1. A defined pathway — Many device makers lack a clear path to improve and develop innovation. Often, innovation occurs in silos, making it difficult for the organization to repeat, sustain, and refine them.
2. Strategic alignment — Lining up product portfolios with corporate strategies is critical to success, yet too often this is not the case in medtech companies. As a result, decision making can become inefficient and ineffective.
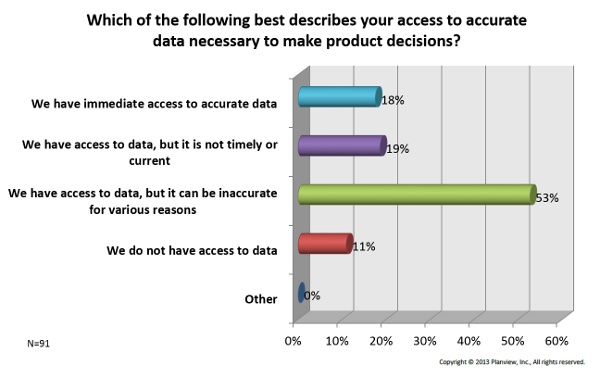
3. Data — Shockingly, over 80 percent of participants said they either have no access to data for decision making, or the data they do have is inaccurate, dated, or difficult to access. See the chart below for the breakdown.
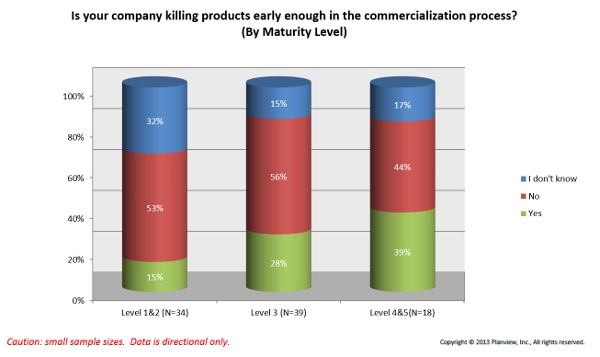
4. Culture — A majority of survey respondents indicated that leadership is averse to risk, and that trend is growing steadily, according to Planview. One way this problem can manifest itself is in a consistent failure to kill off products early or often enough. According to Appleseed’s Carlson, one surveyed company said that by killing off just one product earlier in the commercialization process, it could save approximately $1.2 million.
5 Keys To Innovation Management
In closing, Carlson and Nauyalis shared five recommendations for improving innovation management maturity, based on the findings of the study:
1. The truth will set you free. Some organizations have a culture of lying to themselves. Start by taking a long, honest look at where your company is on the innovation management maturity ladder, where you ultimately want to end up, what challenges you are likely to encounter on the way, and what benefits you can expect to achieve by ascending.
2. Strategically define the destination. You need to look 5 years, or even 10 years, out and set specific goals for your company, division, and brand. As the Planview report states, “There’s no sense in getting somewhere fast if you end up at the wrong destination. Similarly, innovation for innovation’s sake is a waste of money and people.”
3. Three really is the magic number. Nauyalis and Carlson repeatedly referred to the three components of the Innovation Management Maturity Model — people, process, and tools — as the three legs of a stool on which an innovation program sits. You don’t want one leg of your “stool” to be significantly shorter than the others.
4. Culturally encourage innovation. Companies must embrace a culture of (fast) failure in order to succeed. Nauyalis, pointing out that most companies exalt their winners, asked: “Where are the losers? Where are the people who failed fast, who are actually heroes in that they were able to figure out that a product was not going to be a success in the marketplace and quickly killed it? Where are you celebrating these folks?”
5. A call to action from the top. You need to get executive management involved in the process. Changing innovation management maturity is a top-down, not a bottom-up, activity. Leadership needs to be a major proponent of innovation within your organization, providing the vision and the necessary resources.
How does your company rank on the innovation management scale? Where do you think it should be? Perhaps you already know, but if not, you can always take the Innovation Management Maturity Model assessment and download the benchmark report on Planview’s website. (The webinar is also available for replay on YouTube.) Regardless of whether you use tools like these or come up with a framework of your own, you need to make innovation management a priority now — or risk losing opportunities to faster, more organized competition.

