Transient Battery Could Be Adapted To Power Medical Implants
Scientists at Iowa State University (ISU) have introduced what they claim is the first practical transient battery that could be adapted for use in implanted medical devices. Their technology demonstrates the same shelf life and powering capabilities of commercially available batteries, and can dissolve in water in less than thirty minutes.
Next-generation medical implants are getting smaller, doing away with wires and, in some cases, are designed to dissolve when their practical use has expired. According to experts, transient electronics could offer medical devices a host of advantages and avoid complications associated with traditional implants, such as infection, scarring, and risky surgical retrieval.
“The goal of the electronics industry has always been to build durable devices that last forever with stable performance,” explained John Rogers, researcher at the University of Illinois, Urbana-Champaign, during a presentation at the American Chemical Society (ACS). “But many new opportunities open up once you start thinking about electronics that could disappear in a controlled and programmable way.”
Though a lot of progress has been made with electronic circuitry that can dissolve, there has not been as much headway with an effective dissolvable battery, according to Reza Montazami, mechanical engineer at ISU. Montazami commented in a press release that “any device without a transient power source isn’t really transient.”
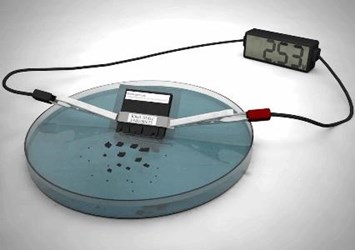
In a proof-of-concept study published in Polymer Physics, Montazami and his team presented their latest development, a lithium-ion battery capable of delivering 2.5 volts of electricity; it could power a desktop calculator for fifteen minutes and dissolve in water in less than 30 minutes. Montazami describes the approach as a “physical-chemical hybrid transiency.”
The ISU team’s battery is made up of eight layers and comprises a more complex structure than had been previously attempted, said the scientists. When placed in water, the polyvinyl alcohol-based polymer that forms the casing breaks apart, and the lithium salt and silver nanoparticles contained inside disperse. While the lithium-based battery prototype is not biocompatible, researchers believe the concept could be applied to medical implants.
“This is a battery with all the working components. It’s much more complex than our previous work with transient electronics,” said Montazami. This technology, he said, is the first of its kind to “demonstrate the power, stability, and shelf life for practical use.”
Researchers commented that future research would be directed towards biocompatibility and designing more controllable systems.
Christopher Bettinger, a biomedical engineer from Carnegie Mellon University (CMU), recently published a study in Trends and Biotechnology in which he stated that “smart” components in medications could dramatically improve their performance and reduce treatment costs. The proliferation of this technology, said Bettinger, will depend on the development of ingestible batteries and circuitry.
Bettinger’s team at CMU designed a transient battery using materials found in trace amounts in the human body — copper, manganese, and magnesium — and their battery provided 5 milliwatts of power for 20 hours.
“The potential impact of ingestible electronics will expand as more materials are added to the tool box,” noted Bettinger.
