World-First Clinical Trial Confirms Efficacy Of Artificial Pancreas
By Chuck Seegert, Ph.D.
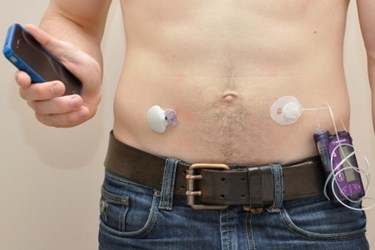
Two configurations of an artificial pancreas were recently compared to conventional diabetes treatments in a world-first clinical trial. Using insulin and glucagon to maintain target blood glucose levels, the devices were demonstrated to achieve significantly greater glycemic control than conventional methods.
Type 1 diabetes is a common disease that tens of thousands of patients are diagnosed with each year. Living with diabetes requires patients to test their blood sugar often and administer insulin with a syringe or a pump. One forgotten injection can lead to life-threatening issues, so great care must be taken.
Recently, a clinical trial performed by researchers at the University of Montréal demonstrated that artificial pancreases outperform conventional types of diabetes treatments. The study was the first of its kind in the world, and it compared three different types of diabetes treatments, including a single-hormone artificial pancreas, a dual-hormone artificial pancreas, and a conventional insulin pump, according to a recent press release from the University of Montréal. The single-hormone system injects insulin, while the dual-hormone version injects insulin and glucagon. Insulin is the hormone that decreases blood sugar levels, while glucagon increases the levels of sugar in the blood.
“Our clinical trial was the first to compare these two configurations of the artificial pancreas with the conventional diabetes treatment using an insulin pump,” said Dr. Rémi Rabasa-Lhoret, an endocrinologist and professor at the University of Montréal’s Department of Nutrition, in the press release. “We wanted to determine the usefulness of glucagon in the artificial pancreas, especially to prevent hypoglycemia, which remains the major barrier to reaching glycemic targets.”
The primary metric that researchers studied was the percentage of times the patient spent in the target plasma glucose concentration window, according to a recent study published by the team in The Lancet Diabetes and Endocrinology. The study also accounted for the number of times patients experienced hypoglycemic events, which are times when blood glucose levels become dangerously low. During the study, conventional insulin pumps led to 52 hypoglycemic events (12 of which were symptomatic), single-hormone artificial pancreases were associated with 13 events (five of which were symptomatic), and the dual-hormone artificial pancreases were associated with nine events (none of which were symptomatic).
Notably, nocturnal hypoglycemic events, which are some of the more concerning as they can lead to coma while a patient sleeps, were also greatly reduced by the artificial pancreases, according to the study. Where the traditional insulin pumps led to 13 nocturnal hypoglycemic events (0 symptomatic), none of the events occurred for patients using artificial pancreases.
“Given that low blood glucose remains very frequent during the night, the fear of severe nocturnal hypoglycemia is a major source or stress and anxiety, especially for parents with young diabetic children,” added Dr. Laurent Legault, a pediatric endocrinologist at Montréal Children’s Hospital and co-author of the study, in the press release. “The artificial pancreas has the potential to substantially improve the management of diabetes and the quality of life for patients and their families.”
Artificial pancreas designs are increasing in many research programs as the concept continues to gain momentum. Recently, another artificial pancreas clinical trial was kicked off in the United States, according to an article published on Med Device Online.
Image Credit: IRCM
