FDA Eases UDI Requirements For Discontinuation Of Legacy Identification Numbers
By Jof Enriquez,
Follow me on Twitter @jofenriq
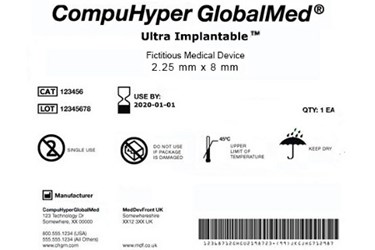
The U.S. Food and Drug Administration (FDA) states in finalized guidance that, in consideration of stakeholder concerns, it does not intend to enforce a planned prohibition against providing National Health Related Item Code (NHRIC) or National Drug Code (NDC) numbers on device labels and device packages until September 24, 2021.
Historically, payers, pharmacies, and retailers selling certain medical devices have used these so-called legacy FDA identification numbers for reimbursement purposes. However, because these legacy numbers can cause confusion regarding appropriate identification of devices, FDA wants industry to stop using these numbers, and instead use a two-part unique device identification (UDI) number under a phased, seven-year plan launched in 2013.
The UDI Final Rule, established in the Food and Drug Administration Amendments Act of 2007 (FDAAA) and implemented in 2013, includes a provision that rescinds any NHRIC or NDC number assigned to a device, according to RAPS.
FDA planned for any NHRIC or NDC numbers to be rescinded as of September 24, 2018. Following a review of 13 comments received for the draft guidance, however, FDA states in finalized guidance that it "does not intend to enforce the prohibition against providing NHRIC and NDC numbers on device labels and device packages, with respect to finished devices that are manufactured and labeled prior to September 24, 2021."
FDA reasoned that it decided to give extra time for compliance because "some affected stakeholders have expressed concern that pharmacies, payers, and other entities are not prepared to transition away from use of NHRIC or NDC numbers in their systems. Some stakeholders have also expressed concern that removal of these legacy FDA identification numbers from device labels according to the timeline required by 21 CFR 801.57 could cause disruption to existing reimbursement, supply chain, and procurement processes."
Pushing back on the deadline means that there will now be prolonged period of time when multiple identifiers exist on medical device labels, creating more confusion. But FDA says the risk of confusion is "outweighed by the threat of disrupting reimbursement, supply chain, and procurement processes and potentially interfering with patient access to devices." The agency says it's better to set a consistent date by which all classes of devices have legacy NHRIC and NDC numbers removed.
In addition, FDA intends to consider requests for continued use of an FDA labeler code under a system for the issuance of UDIs until September 24, 2021. FDA also won't take action against a labeler for incorporating a previously assigned FDA labeler code into its UDI without requesting approval to do so by the deadline.
"We expect the UDI labeling requirements will be fully implemented by September 24, 2021. We also believe additional time is appropriate for stakeholders to adopt medical device reimbursement, supply chain, and procurement systems, which do not depend on having an NHRIC or NDC number on the device label," states the FDA's notice in the Federal Register.
