FDA Hikes Medical Device User Fees For FY 2016
By Jof Enriquez,
Follow me on Twitter @jofenriq

The U.S. Food and Drug Administration (FDA) is raising medical device user fees for fiscal year 2016. The new rates will take effect starting Oct. 1, 2015 and remain static through Sept. 30, 2016.
Under the Federal Food, Drug, and Cosmetic (FD&C) Act, as amended by the Medical Device User Fee Amendments of 2012 (MDUFA III), the FDA is allowed to collect user fees for medical device submissions or applications in order to supplement the agency's federally-appropriated funding. In exchange, the FDA is expected to use the additional revenue from user fees to conduct more efficient, timely, and transparent reviews. The agency adjusts the rates annually, taking into consideration factors like inflation, staffing costs, and regulatory workload.
According to the official notice from the Federal Register, “the total revenue amount for FY 2016 is $129,339,949, as set forth in the statute prior to the inflation adjustment. (See 21 U.S.C. 379j(b)(3)(D)). MDUFA III (Pub. L. 112-144) directs FDA to use the yearly total revenue amount as a starting point to set the standard fee rates for each fee type.”
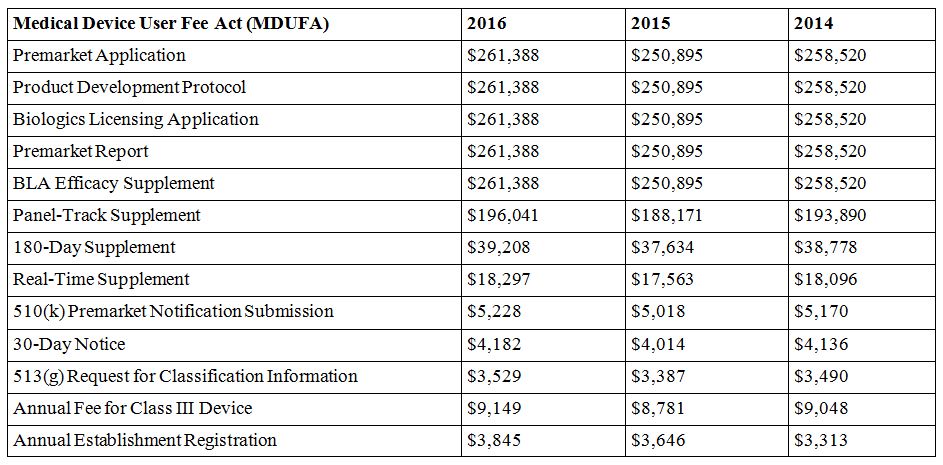
The standard fee for a premarket application, product development protocol, biologics licensing application, premarket report, and BLA efficacy supplement, is set at $261,388 each for FY 2016. Fees for other submissions are calculated as varying percentages of that amount.
Based on aforementioned factors such as inflation, user fees were increased in 2014, lowered in 2015, then raised again for the next fiscal year. Specifically, premarket applications (PMAs) for medical devices "increased from the last fiscal year to this one by 4.2 percent, but from the 2014 to 2015 fiscal year it actually went down by 2.9 percent," notes Med City News.
RAPS provides a year-by-year comparative table of MDUFA user fees.
User fees have been subject to federal sequestration since the U.S. Congress passed the Budget Control Act in 2011, although the FDA has recovered some sequestered funds.
According to AdvaMed, “The Omnibus Appropriations legislation passed in January 2014 retroactively restored $2.85 million of sequestered user fees to FDA from FY 13, and the Murray-Ryan budget deal eliminated threatened cuts for FY14 or FY15. However, going forward into FY16 and beyond, additional medical device user fees are at risk since sequestration cuts could come into effect once more.”
AdvaMed is lobbying for additional legislation, such as the FDA SOS Act, to exempt FDA user fees from sequestration measures.
