FDA Releases Draft Guidance On Demographic Data In Clinical Trials
By Jof Enriquez,
Follow me on Twitter @jofenriq

The U.S. Food and Drug Administration (FDA) has issued draft guidance for the evaluation and reporting of age, race, and ethnicity data in medical device clinical studies, which should reflect representative proportions of intended use populations.
FDA's Center for Devices and Radiological Health (CDRH) had previously committed to develop the draft guidance under Section 907 of the FDA Safety and Innovation Act of 2012 (FDASIA) in order to improve the completeness, quality, and public availability of demographic subgroup data from medical device clinical studies.
FDA states that it sought expert and stakeholder input, through various public for a, to help develop the recommendations set forth in the draft guidance, Evaluation and Reporting of Age, Race, and Ethnicity Data in Medical Device Clinical Studies, which has three objectives:
- Encourage the collection and consideration during the study design stage of relevant age, race, ethnicity and associated covariates (e.g., body size, biomarkers, bone density, etc.), for devices for which safety, effectiveness (probable benefit, for HDEs), or benefit-risk profile is expected to vary across these groups;
- Outline recommended analyses of study subgroup data, with a framework for considering demographic data when interpreting overall study outcomes; and
- Specify FDA’s expectations for reporting age, race, and ethnicity-specific information in summaries and labeling for approved or cleared medical devices.
The draft guidance is included in FDA's “B-list,” or the list of guidance documents intended for publication as FDA's resources permit.
FDA plans to integrate the draft guidance’s content and that of a previous guidance, Evaluation of Sex-Specific Data in Medical Device Clinical Studies, into one final guidance document. The final guidance also will extend and complement another guidance, Collection of Race and Ethnicity Data in Clinical Trials.
The Regulatory Affairs Professional Society (RAPS) notes that a substantial number of clinical studies, in recent years, lacked diverse demographic information. A 2013 FDASIA report showed that only 40 percent of pre-market approvals (PMAs) reported an age-based analysis of outcomes data, only 27 percent included a race or ethnicity subgroup analysis, and just 16 percent had public statement regarding a race or ethnicity analysis, according to RAPS. This has clinical implications, because studies of medical devices should not use a one-size-fits-all approach.
“For example, the use of cochlear implants in certain pediatric subgroups may not be advisable due to the size of the implant, or may be inappropriate due to the stage of the neurological development of the child. In the case of intraocular lenses used to treat vision loss, device use may also improve future visual development in a young child,” FDA said in the draft guidance.
According to FDA, historically, many medical device clinical studies have not enrolled proportions of age, race, and ethnic subgroups that reflect the underlying disease distribution in the affected population, which can be "problematic because the ability to detect differences in response to treatment is markedly diminished if there is no or limited clinical experience with the product in the subgroup of interest."
"It is important that clinical trials include diverse populations that reflect the intended population, especially when clinically meaningful differences in safety, effectiveness, (probable benefit, for HDEs), or benefit-risk profile are expected across these groups," said FDA.
In the guidance, FDA offers several recommendations on how to increase enrollment of underrepresented groups based on age, race, and ethnicity during study planning, including the identification of the following information in the study protocol and submission documents:
- Age, race, and ethnicity-specific prevalence, if known;
- Age, race, and ethnicity-specific diagnosis and treatment patterns, if known;
- Proportions of age, race, and ethnicity subgroups included in past studies for the target indication, if known; and
- Any known clinically meaningful age, race, and ethnicity-specific differences in outcomes related to either safety or effectiveness (or probable benefit for HDEs).
FDA recommends that sponsors and clinical study investigators consider methods to help avoid or minimize loss-to-follow-up of subjects (regardless of age, race, or ethnicity subgroup), including developing a "follow-up plan that details follow-up goals, frequency of upcoming scheduled follow-up visits, proxy contact information, and number and type of contacts for patients missing a follow-up visit" and "participating in cultural competency training prior to study recruitment."
FDA reminds clinical trial sponsors to consider in their study design, analysis, and interpretation of study results certain "intrinsic and extrinsic biological differences across age, race, and ethnic groups (e.g., gonad development, skin texture, skin color, hormone levels, metabolism, degenerative disease, bone density, cell receptors, etc.) that may influence the safety and effectiveness (or probable benefit for HDEs) of a device."
"For example, ionizing radiation exposure to pediatric patients from medical imaging procedures is of particular concern because pediatric patients are more radiosensitive than adults (i.e., the cancer risk per unit dose of ionizing radiation is higher)," said FDA.
Cognizant of differences in device safety and effectiveness across age, race, and ethnic subgroups, FDA enjoins sponsors to investigate heterogeneity across demographic subgroups, especially for primary safety and effectiveness endpoints. FDA also offers recommendations on how to integrate subgroup specific statistical elements.
FDA acknowledges that labeling and FDA summaries of review for approved medical devices in the past may be inconsistent with regards to device performance in demographic subgroups. Therefore, the agency encourages sponsors to submit and publicly report the number and proportion of subjects by age, race, and ethnic groups who were treated or diagnosed with a device as part of a clinical study. FDA offers example language that sponsors can use in their reports, which may contain only conclusions based on factual data, rather than assumptions or inferences. FDA added that "outcomes analyses by demographic subgroup should be reported in the labeling and review summaries."
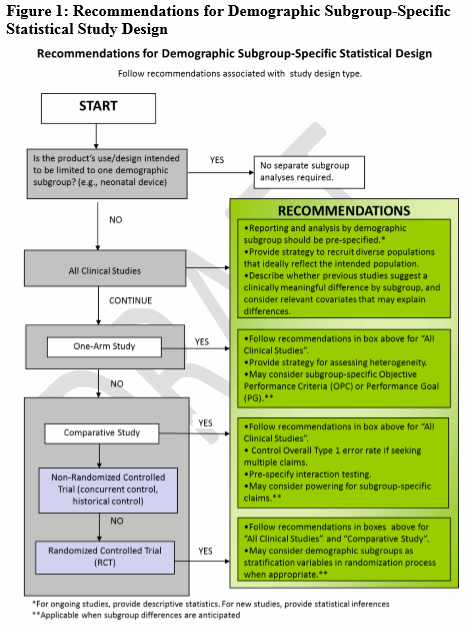
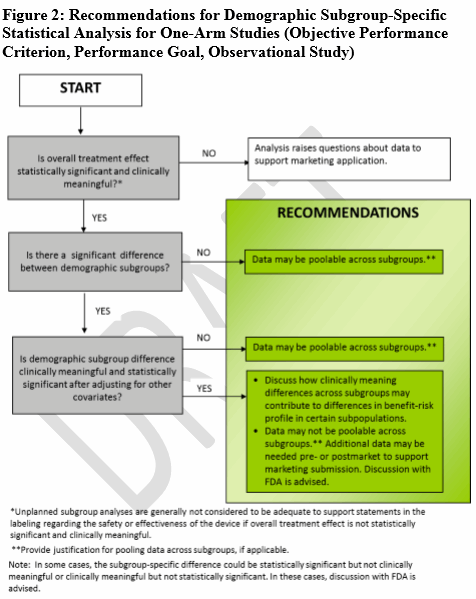
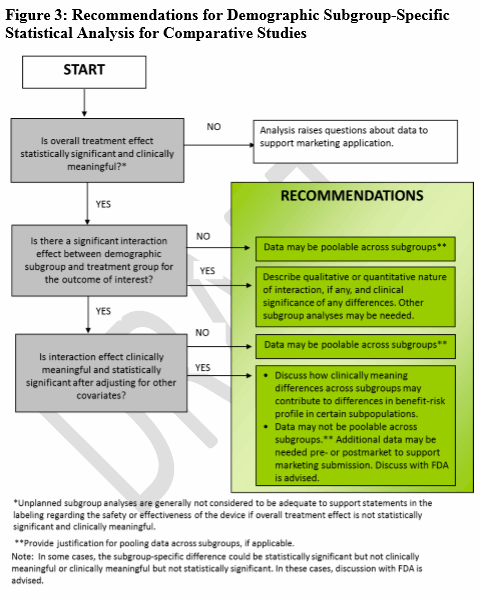
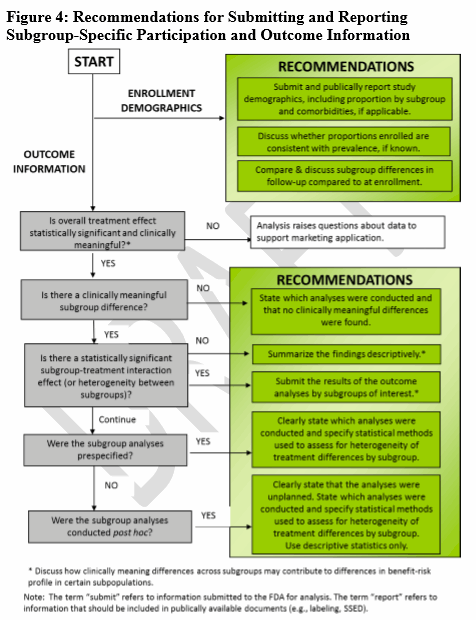
When there is a hypothesis for a clinically meaningful difference (through the use of recent previous studies, disease natural history studies), FDA offers four decision trees (Figs. 1-4) to guide sponsors in deciding when various age, race, or ethnicity-specific statistical recommendations apply for different clinical study designs (i.e., statistical study design, one-arm studies, comparative studies, participation, and outcome information).