J&J's Biosense Acquires WaveCrest LAA Closure Device Developer
By Jof Enriquez,
Follow me on Twitter @jofenriq
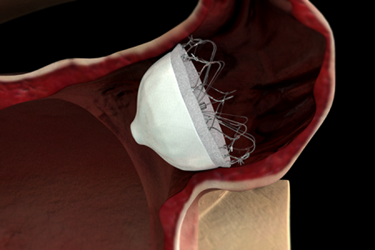
Johnson & Johnson subsidiary Biosense Webster has purchased Coherex Medical, maker of the WaveCrest left atrial appendage (LAA) occlusion device for the treatment of high-risk atrial fibrillation (AF), for an unspecified amount.
"The Coherex WaveCrest System offers substantial benefits for patients with atrial fibrillation who are at high risk for stroke, particularly for those who are contraindicated to anticoagulants and would therefore, be unprotected from the risk of cardio embolism," said Alex Martin, president and CEO, Coherex Medical, in a statement. "We are excited that this technology will be coupled with Biosense Webster’s market leading therapies and look forward to bringing this innovative solution to more patients worldwide who stand to benefit from reduced risk of stroke.”
In patients with atrial fibrillation, the upper chambers of the heart quiver instead of contracting rhythmically, which predispose the patient to formation of stroke-causing clots. The majority of these clots are thought to be formed in the LAA, a small pouch attached to the left atrium. Permanent closure of the LAA could therefore reduce the overall risk of strokes, especially for patients with AF who are contraindicated or dislike anti-coagulant therapy.
“The addition of the Coherex WaveCrest System complements our comprehensive portfolio of therapeutic solutions for patients suffering from atrial fibrillation who not only suffer from reduced quality of life but also face a significantly greater risk of a stroke,” added Shlomi Nachman, worldwide president, Biosense Webster, and company group chairman, Johnson & Johnson, in the statement. "As the exclusive distributor of this system in regions outside of the U.S. since 2013, we are confident it will be well-differentiated in the market.”
The Wavecrest System received CE Mark in September 2013, but is not yet available for investigational use or commercial distribution in the United States. It is similar to St. Jude Medical’s Amplatzer Amulet endocardial LAA closure device, which also has received CE Mark approval, and Boston Scientific's Watchman device, whose FDA approval earlier this year has prompted a surge in enthusiasm in this device segment.
According to iData Research, the increasing prevalence of AF and rising penetration rate of more advanced device-based LAA closure procedures, versus traditional surgical closure of LAAs, is expected to drive growth in the U.S. market for LAA closure devices through the remainder of the decade.
While saying that additional clinical studies are needed, medical societies have acknowledged the potential of device-based closure in preventing strokes in the approximately 2.7 million Americans who experience AF.
“The implantation of left atrial appendage occlusion devices may lower the risk of stroke in patients with atrial fibrillation. As new devices are developed, it is anticipated that the use of left atrial appendage occlusion technologies in clinical practice will expand,” states a joint overview released in June by The American College of Cardiology, Heart Rhythm Society, and the Society for Cardiovascular Angiography and Interventions.
