Medtronic Announces Foray Into Wearable Tech For Mental Health
By Jof Enriquez,
Follow me on Twitter @jofenriq
Medtronic is collaborating with Australian medtech company Medibio on a non-invasive, wearable device-based solution to diagnose depression and other mental conditions using electrocardiogram (ECG) and circadian heart rate variability analysis.
Medibio announced recently a non-binding memorandum of understanding (MOU) with Medtronic in which the two companies will talk about a potential "strategic agreement covering business opportunities and synergies across both companies."
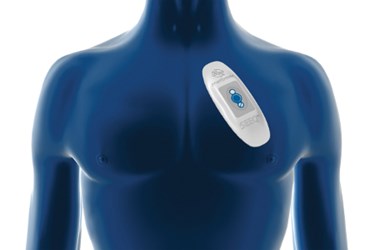
Sydney-based Medibio — which has ties to Minnesota, where Medtronic's operational headquarters is located — is looking to utilize Medtronic's expertise in medical devices, particularly the heart-monitoring patch called Seeq, reports the Minneapolis/St. Paul Business Journal. The wearable tech was acquired by Medtronic through its purchase of Corventis in 2014.
Seeq is an adhesive, wearable sensor that can be worn for up to 30 days to help detect and diagnose the cause of irregular heartbeats in patients. It automatically sends data via Bluetooth and cellular signals to the Medtronic Monitoring Center, where reports are processed and submitted to cardiologists to aid diagnosis. It’s a shorter-term monitoring solution to complement Medtronic's LINQ implant, which can monitor cardiac rhythm for three years.
Medibio has developed a system where biometric data from next-generation wearables, such as Seeq, can be pre-processed via a smartphone app before being sent to the cloud. Medibio claims its cloud-based proprietary machine learning algorithms can correlate thousands of circadian heart rate (CHR) variability waveforms
According to a company presentation, the research behind its technology has been validated both internally and externally via blinded studies, with one external blinded study peer-reviewed and published showing between 78 and 92 percent diagnostic accuracy.
A proprietary depression-test will be the first-ever quantitative diagnostic test in the field of mental health, according to Medibio, which plans to obtain FDA clearance by submitting a de novo 510(k) premarket notification application.
Besides talking with Medtronic, Medibio inked an MOU with Preventice, which also makes a cardiac-monitoring patch for real-time ECG monitoring out of the Twin Cities area.
Related, a Silicon Valley medtech startup has developed technology similar to Medibio's system. Santa Clara, Calif.-based VivaLnk makes the Vital Scout patch with multiple sensors that track heart rate and heart variability, respiration rate, stress levels and stress recovery, sleep status, and activity/training status. The device transmits data to iOS and Android devices via Bluetooth.
With the wearable device market projected to surge to $41 billion by 2020, device giants and medtech startups alike are looking to take advantage, especially as preventive and remote healthcare monitoring become more common.
