Medtronic, Edwards, LivaNova Tout Valve Studies At ACC
By Jof Enriquez,
Follow me on Twitter @jofenriq
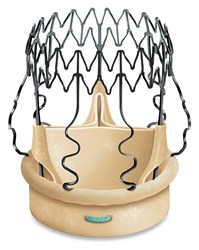
Medtronic, Edwards Lifesciences, and LivaNova presented favorable results of their respective heart valve clinical trials at the 65th Annual Scientific Session and Expo of the American College of Cardiology (ACC) in Chicago. These studies focused mostly on transcatheter heart valve technologies, which are primed for increased adoption and growth.
According to Medtronic, the High Risk Study of the CoreValve U.S. Pivotal Trial showed that its self-expanding CoreValve transcatheter aortic valve replacement (TAVR) device delivered superior clinical outcomes compared to surgical aortic valve replacement (SAVR) out to three years.
"It's reassuring to see that the CoreValve High Risk Study continues to show that TAVR is superior to surgery at three years for the combined endpoint of mortality and stroke, which has the most important impact on patients," said G. Michael Deeb, M.D., Herbert Sloan Collegiate Professor of Cardiac Surgery, University of Michigan Frankel Cardiovascular Center, in a statement. "Most importantly, the new data supports the viability of TAVR out to three years with no signal of a significant increase in mean gradient or aortic regurgitation."
In addition, a sub-study analysis of the High Risk Study in patients with an STS Predicted Risk of Mortality estimate <=7 percent demonstrated superior outcomes in all-cause mortality at two years compared to patients treated with SAVR, and superior rates of combined all-cause mortality or major stroke, according to Medtronic.
CoreValve is a self-expanding valve ideal for patients with exceptionally small vasculature, and was the first transcatheter heart valve approved in the United States for valve-in-valve procedures in both high- and extreme-risk patients with failed surgical valves. It has shown positive clinical outcomes at one year in new patient populations with significant co-morbidities (end-stage renal disease, low gradient aortic stenosis). Its newest iteration, the CoreValve Evolut R System, is the first recapturable and repositionable TAVR system offered in the U.S.
Medtronic also presented early feasibility study results of its Harmony Transcatheter Pulmonary Valve (TPV), which is designed as a first-of-its-kind minimally invasive alternative to open-heart surgery for patients with congenital heart disease (CHD). Medtronic is positioning Harmony as a less invasive option for approximately 80 percent of patients who currently undergo initial open heart surgical valve replacement to restore normal valve function. As a follow-up, the company is planning an investigational device exemption (IDE) study sometime in late 2016.
Also at the ACC meeting, Edwards Lifesciences touted that the randomized PARTNER II Trial, which compared the SAPIEN XT valve to surgery in intermediate-risk patients, successfully achieved its primary endpoint at two years.
"The PARTNER II Trial is unique in size, distinctive in rigor of execution and exceptional in patient outcomes," said Craig Smith, M.D., chair, Department of Surgery, and surgeon-in-chief, New York-Presbyterian Hospital/Columbia University Medical Center, as well as co-principal investigator of the PARTNER II Trial, in a statement. "In intermediate-risk patients, transcatheter and surgical valve replacements were similar with respect to the combination of mortality and disabling stroke. In this study, TAVR significantly shortened ICU and hospital stays, and, in patients who are candidates for transfemoral access, the SAPIEN XT valve may have additional clinical advantages."
London-based medical device manufacturer LivaNova announced the first implant of the Perceval sutureless valve in the PERSIST-AVR (Perceval Sutureless Implant Vs Standard Aortic Valve Replacement) trial, which is the first prospective, randomized study in 30 years in the field of valvular surgery. Comprising participants from more than 60 centers worldwide, the trial will compare LivaNova's Perceval sutureless aortic valve with standard sutured bioprostheses in 1,200 patients suffering from severe symptomatic aortic stenosis, or steno-insufficiency in patients who are candidates for surgical replacement of their native aortic valve.
"As the first trial of its kind, the PERSIST-AVR study is a significant milestone for LivaNova, the healthcare community and patients worldwide,” said Michel Darnaud, president, Cardiac Surgery B.U., LivaNova, PLC, in a statement. “We are proud to support this important trial. We anticipate its results could significantly impact daily practice and establish the Perceval sutureless valve as the prosthesis of choice for future heart valve surgeries.”
