Stryker Adds SafeWire MIS Products To Spine Portfolio
By Jof Enriquez,
Follow me on Twitter @jofenriq
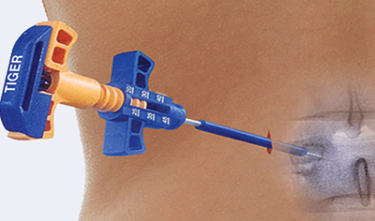
Stryker Corp. has acquired Florida-based medical device startup SafeWire's Y-Wire guidewire and Tiger Jamshidi Needle Family portfolio to bolster its offerings for minimally-invasive spine (MIS) surgery.
Inadvertent migration of pedicle screw guidewires is a known risk in MIS surgery, potentially causing visceral or vessel damage. The Y-Wire guidewire, with its distinctive bifurcated tip, is designed to limit this risk by preventing inadvertent advancement through the bone. The Nitinol shape-memory alloy is designed to ensure Y-Wire’s tip retains its open configuration, thereby minimizing the risk of kinking or accidental pullouts. With these advantages, the use of Y-wire guidewire offers fewer surgical steps and greater technical ease compared to standard guidewires, and it decreases patient and surgeon exposure to fluoroscopy, according to Stryker. SafeWire received CE Mark certification to commercially release Y-Wire in October 2014.
The Tiger needles are used for MIS percutaneous posterior fixation surgery. It’s a device designed for threaded advancement of needle through bone, which gives surgeons optimal control and reduces risks linked to impact advancement of pedicle screw needles, such as unintended trajectories or sub-optimal needle depth. It was granted 510(k) clearance from the U.S. Food and Drug Administration (FDA) in June 2014, and commercially launched in the U.S. in September 2014.
Stryker said the SafeWire portfolio acquisition is "highly complementary" to its current spine product portfolio, and is aligned with the Spine division’s strategy of expanding its product offering for MIS.
“We are excited to bring this important complementary technology into the Stryker family,” Brad Paddock, president of Stryker’s Spine division, said in a press release. “This acquisition increases our competitive advantage as we broaden our product line and extend our customer base among teaching facilities, competitive accounts, and existing SafeWire customers.”
The acquisition is the latest in a recent string of deals made by Stryker. The medical device maker closed a trio of large deals in February, buying hospital supplies provider Sage Products for $2.78 billion, emergency medical services (EMS) equipment manufacturer Physio-Control for $1.3 billion, and Synergetics’ neurology portfolio for an undisclosed sum.
Stryker CEO Kevin Lobo told analysts that the company will be active on the M&A front in the foreseeable future, one of the reasons it postponed its repurchase program, reports Reuters.
Lobo said during Stryker’s Q4 2015 earnings call that the company's Spine business posted its third consecutive quarter of strong growth, with sales registering mid-single digit percentage growth in constant currency in the last reporting quarter, and showing high single-digit growth in the U.S. He was optimistic that the Spine business will continue its upward trend with the introduction of a new 3D-printed titanium interbody device sometime in 2016.
