TransEnterix Gears Up For Senhance Robot's Wider Commercialization, FDA Approval In 2017
By Jof Enriquez,
Follow me on Twitter @jofenriq
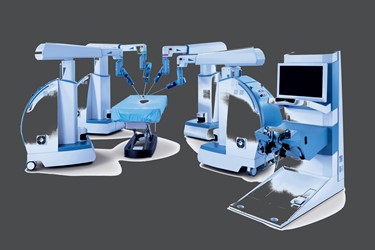
Research Triangle Park, N.C.-based TransEnterix says it intends to file the 510(k) application for its Senhance Surgical Robotic System in early 2017, with FDA clearance expected also next year. In anticipation of wider commercialization of the platform, the company has inked a clinical partnership with Imperial College London, as well as a distribution deal with Getz Healthcare.
Company president and CEO Todd Pope told analysts that the priorities for 2017 were, "First, continue to commercialize the Senhance platform in CE mark countries through a combination of direct resources and distributors. Second, partner with leading hospitals and surgeons through our clinical leadership program, third obtain US regulatory 510(k) clearance for the Senhance System and fourth, leverage the open architecture of the Senhance platform," according to a Seeking Alpha transcript.
TransEnterix is one of a handful of new players looking to challenge Intuitive Surgical as the leader in the market for robot-assisted surgery. While Intuitive's da Vinci robot dominates the high-end segment, other companies like TransEnterix are looking to compete in the low- to mid-range market of hospitals that are open to robotics, but also are tightening their belts.
In September 2015, TransEnterix bought the surgical robotics division of Italian company SOFAR, including the CE-marked ALF-X robot for minimally invasive surgery. It later rebranded it from ALF-X to Senhance, which was used recently for its first radical hysterectomy.
While Intuitive's system requires surgeons to sit behind a fixed console at a distance, Senhance lets the surgeon operate in the sterile field beside the patient, while providing haptic and tactile feedback and eye-sensing camera control. TransEnterix believes these key differentiators, along with lower pricing, will let it compete and increase penetration among hospital clientele.
Pope told analysts that the company has conducted more than 100 hands-on evaluations with surgeons of the Senhance platform at the European Society of Gynecologic Endoscopy in Brussels, Belgium, The French Multidisciplinary Oncology Meeting in Marseille, and the American College of Surgery in Washington, D.C., as well as over 70 mobile demonstrations at events in London, Paris, Dusseldorf and Munich.
This week, the company installed a Senhance unit in partnership with Imperial College London and St. Mary’s Hospital of the Imperial College Healthcare NHS Trust. Imperial will use the Senhance robot in their minimally invasive surgery program in general, bariatric and colorectal surgery.
“We are pleased to be the first center in the UK to use this new robotic technology,” said Professor Lord Ara Darzi, The Paul Hamlyn Chair of Surgery at Imperial College London, in a news release. “Our centre thrives on combining promising technology with our skilled consultant surgical team to advance the field of minimally invasive surgery and we look forward to developing this program together.”
In addition to Imperial College, TransEnterix also said it plans to establish two to three additional clinical leadership sites over the next two quarters for their surgeon evaluation program.
TransEnterix this week also inked a distribution deal with Getz Healthcare to be the exclusive distribution partner for the Senhance in the Australian and New Zealand markets.
“We chose to partner with TransEnterix because of the tremendous value proposition that the Senhance provides to patients and healthcare providers. Hospitals today are seeking a new way to utilize robotics in surgery that allows them to better manage operational costs," said James Simkins, CEO of Getz Healthcare.
Apart from Getz, TransEnterix also has distributors in Taiwan, Kuwait, and the United Arab Emirates. The majority of these distribution agreements include minimum system purchase obligations in 2017 and/or 2018, according to TransEnterix.
For the third quarter ended Sept. 30, 2016, TransEnterix reported revenue of approximately $1.5 million as a result of the sale of one Senhance System and related instruments.
Pope made it clear that the company's top regulatory priority remains to submit a 510(k) for the Senhance as they prepare for full commercialization. But he adds that TransEnterix intends "to evaluate a SurgiBot resubmission" following FDA's April denial of marketing clearance for the company's first surgical robot.
