FDA Allows St. Jude To Resume Transcatheter Heart Valve Trial
By Jof Enriquez,
Follow me on Twitter @jofenriq
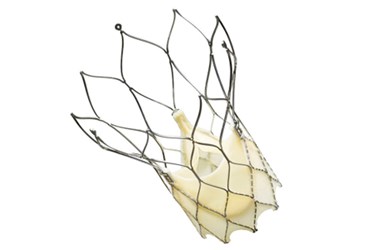
St. Jude Medical has received approval from the United States Food and Drug Administration (FDA) to resume the investigational device exemption (IDE) trial evaluating its Portico Transcatheter Aortic Valve Implantation System after internal and independent reviews of the previously halted study showed no adverse events associated with the implants. The study is designed to support the device's approval application with the FDA.
The company suspended implants of the valve worldwide in September 2014 after reduced leaflet motion was reported in some participants. St. Jude's subsequent investigation indicated that the reports did not amount to excess adverse clinical events, did not affect valve clinical performance, and could be resolved through medical therapy. Independent reviews mirrored St. Jude's findings, determining that the observations of reduced leaflet mobility were a class effect, common among other commercially available transcatheter aortic valve replacement (TAVR) valves, which were used as the study’s control arm.
“We are excited to resume our U.S. IDE trial, which is a critical step toward providing the Portico valve to more patients in need,” said Dr. Mark Carlson, chief medical officer for St. Jude Medical, in a press release. “We have been encouraged by the clinical results and the response of physicians to the Portico valve in Europe and other geographies. We look forward to continuing to partner with physicians in the U.S. to further evaluate the benefits of the Portico system.”
St. Jude claims that the Portico system's unique design makes implantation of the device simpler and safer than current TAVR models. In a previous statement, describing the first implantation of Portico devices in the U.S. last year, St. Jude described the Portico device as the first aortic heart valve “that can be completely resheathed (the process of bringing the valve back into the delivery catheter), repositioned at the implant site, or retrieved before being released from the delivery system.”
In the recent press release, Dr. Raj Makkar, director of interventional cardiology at Cedars-Sinai Heart Institute in Los Angeles and a co-principal investigator for the Portico U.S. IDE trial, said “I’m very proud to work alongside a team of investigators that partnered with St. Jude Medical to prioritize patient safety and support the industry’s current understanding of leaflet motion observations relative to transcatheter and surgical valves. I’m excited to be able to resume our IDE study and advance the evaluation of the Portico valve in the U.S.”
The Portico U.S. IDE trial is being conducted to assess the safety and effectiveness of the Portico device in helping patients with aortic stenosis who are considered high risk for an open-heart valve replacement procedure. St. Jude says that, with the minimally invasive Portico, more patients who were previously ineligible for surgery can now receive treatment. The device already has received CE-mark clearance in Europeand could be eligible to receive FDA clearance, pending results of the IDE clinical trial, which is due for completion before August 2019.
Other medical device manufacturers, including Medtronic (CoreValve) and Edwards LifeSciences (SAPIEN 3), also have presented promising results from their own TAVR clinical trials recently.
