J&J, S&N Lead $7 Million Financing Into OrthoSpace
By Jof Enriquez,
Follow me on Twitter @jofenriq
Johnson & Johnson (J&J) and Smith & Nephew (S&N) led investors pouring $7 million in equity funding into OrthoSpace, which intends to use the money to support its ongoing U.S. Investigational Device Exemption (IDE) trial and commercialization activities.
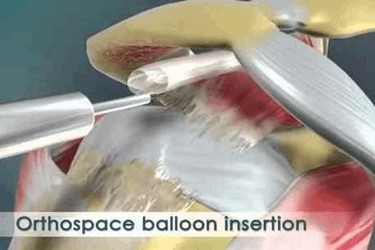
OrthoSpace develops and manufactures InSpace, a CE-marked, minimally-invasive, biodegradable balloon system for the treatment of rotator cuff injuries. InSpace acts as a spacer between the acromion and the humeral head to allow smooth articulation between the two bones to mimic the function of the original bursa, thereby reducing shoulder pain, increasing range of motion, and shortening the rehabilitation process.
More than 10,000 InSpace balloons have been implanted in outpatients across Europe, Israel, Russia, and Hong Kong, according to the orthopedics startup based in Caesarea, Israel.
"This is an exciting time for OrthoSpace," Itay Barnea, OrthoSpace CEO, said in a news release. "We are seeing great traction for InSpace among the European shoulder community where surgeons are grateful for a simple, effective option to treat rotator cuff injury. US surgeons have begun to learn about and gain experience with our device through participation in the US IDE Study, where enrollment is well underway. We are pleased to complete this financing to continue to invest in the Company's growth, and we look forward to offering InSpace in additional major markets worldwide."
The $7 million second round of financing for OrthoSpace was participated in by J&J, along with previous investors HealthpointCapital, S&N, and TriVentures, who led the initial round of financing worth $8 million announced in May 2015 to support the IDE study, according to CrunchBase.
The ongoing U.S. IDE trial is a 184-patient randomized, multi-center, single-blinded control study that compares the InSpace biodegradable balloon system to conventional repair or partial repair for the treatment of full thickness massive rotator cuff tears.
OrthoSpace was founded in the start-up incubator of Xenia Venture Capital as a BioProtect spin-off, which had developed a balloon to separate healthy tissue from an enlarged prostate. That technology was later repurposed for treating rotator cuff tears, according to Israeli publication Globes.
Rotator cuff tears are one of the most common orthopedic injuries, especially for those over the age of 40. They are caused by traumatic injuries, repetitive use of the shoulder joint, and degenerative changes.
