MHRA Issues Pre- And Postmarket Clinical Trial Design Recommendations For Leadless Pacemakers
By Jof Enriquez,
Follow me on Twitter @jofenriq

The United Kingdom (UK) Medicines and Healthcare Products Regulatory Agency (MHRA) has released initial recommendations for pre-market and postmarket clinical evaluation of leadless cardiac pacemakers.
MHRA acknowledges that innovative leadless pacing technology is "a major departure," rather than merely an iteration of conventional cardiac rhythm management (CRM) devices, and that CE marking has been given to leadless pacemakers based on limited clinical data at the time of approval, as commonly practiced in the EU.
To date, these devices are: St. Jude Medical's Nanostim, Medtronic's Micra, and EBR Systems' Wireless Cardiac Stimulation system (WiCS), according to RAPS. Medtronic's Micra also has been approved by the U.S. Food and Drug Administration (FDA).
While MHRA says it promotes innovative medical technologies like leadless pacing, and that the CE Marking process generally is faster than FDA’s process in the U.S., the U.K. agency also "would like to see clinical guidance on the use of leadless pacemakers combined with robust surveillance of safety, to ensure that this new technology is used appropriately and that the risk of patient harm is as low as possible."
For this reason, an Expert Advisory Group was convened in 2015 by the MHRA’s Devices Expert Advisory Committee (DEAC) to develop a framework document for manufacturers and notified bodies to follow in preparing the clinical evaluation of leadless pacemakers before and after these devices hit the European market.
MHRA’s guidance document contains initial recommendations for adoption of leadless cardiac pacing therapy, including requirements for selection of patients and centres, and minimum acceptable operator experience and training. It also gives guidelines on how manufacturers should perform implant surveillance, including components of a post-market clinical follow-up (PMCF) study or registry.
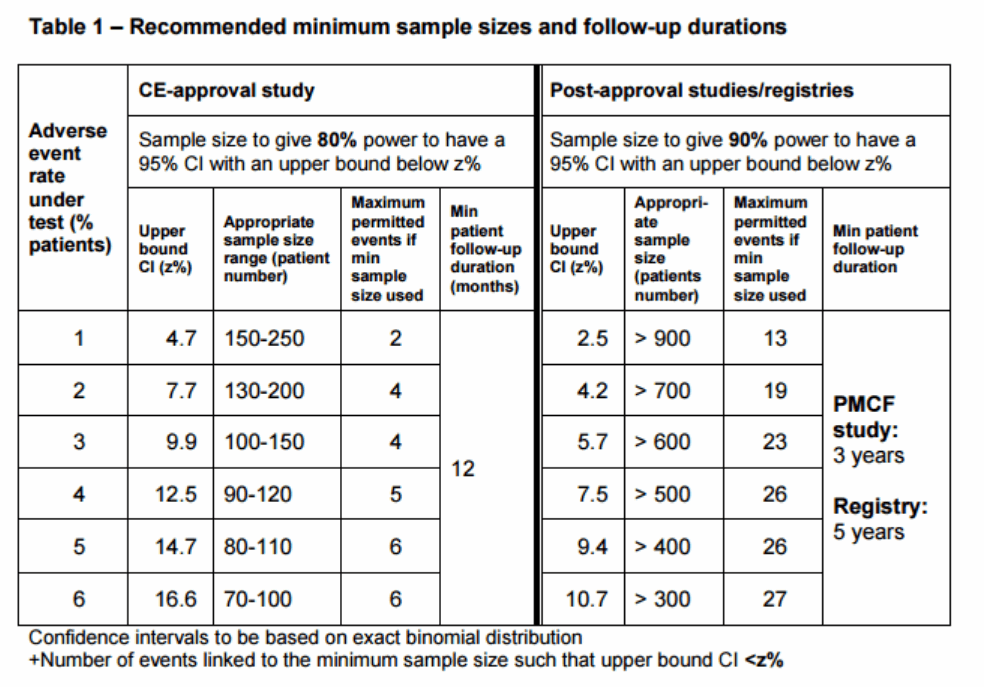
The document lays out the recommended design of clinical studies for EU market approval and post-market follow-up of leadless cardiac pacing therapy. This includes advice on general statistical principles which can guide decision making in the study conduct and design, including appropriate representative sampling methods, as well as ideal sample size, confidence intervals, and follow-up duration.
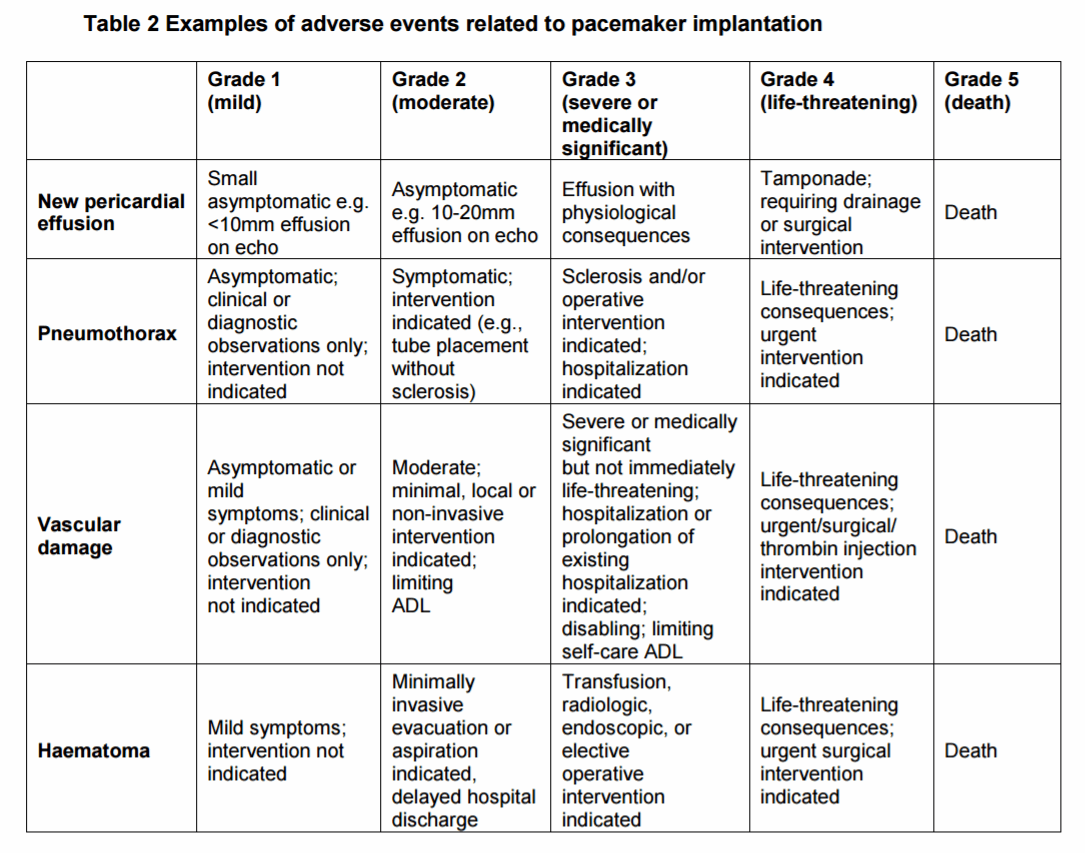
MHRA also gives some examples of the types and grade of severity of adverse events.
MHRA states: For the statistical assumptions used in data analysis to be appropriate, it is essential that the study design is agreed in advance with regulators and not altered without reassessment of the statistical design. Such studies can, at best, explore adverse event rates associated with short or medium-term implant duration. Therefore, they should, except in exceptional circumstances, be supplemented by PMCF studies or registries to evaluate the longer-term safety and performance of the new or novel treatment. PMCF study and registry requirements should also be based on learnings during pre-market evaluation.
The agency says the guidance document will be updated periodically as evidence for other leadless CIED (cardiac implantable electronic device) technology increases.
According to Erik J. Bracciodieta, a Senior Analyst on the Medical Device Insights team at Decision Resources Group, leadless pacemakers “will be able to command a premium price even greater than MRI-compatible devices, leading to market growth even as pacemaker unit sales decline in developed countries.”
