An Overview of the 5 Types Of Neuromodulation Devices
By Kateryna Hronska and Kamran Zamanian, Ph.D., iData Research Inc.
Electrical and chemical stimulation have been used to treat neurological symptoms for over 60 years. Mainly, the technique is used to treat pain, movement disorders, spasticity, epilepsy, stupor and coma psychiatric disorders, neurogenic bladder, peripheral vascular insufficiency, and diaphragmatic palsy.1 Therapy can be delivered by electrically stimulating nerves or by targeted drug therapy.1 This article provides an overview on spinal cord stimulation (SCS), deep brain stimulation (DBS), sacral nerve stimulation (SNS), vagus nerve stimulation (VNS), and gastric electric stimulation (GES), as well as related market trends.
Spinal Cord Stimulation (SCS)
SCS devices deliver electrical pulses to the spinal cord to relieve chronic pain. They are often used as treatment for failed back surgery syndrome, injuries to the spinal cord, complex regional pain syndrome, neuropathic pain, and ischemia-related refractory pain. The main benefits of the therapy are the decreased need for pain medication and overall improvement of the quality of life.
An SCS system consists of a pulse generator and usually two leads, where the electrodes at the end of the leads are arranged to overlap the region of pain. It is now standard for leads to have eight electrodes each, where one to four leads can be installed, depending on the specifications of the generator. The lifespan of rechargeable neurostimulators will depend on the energy consumption of the device. However, they typically last for seven to nine years; some can last up to 20 years and primary cell neurostimulators will typically last for five to seven years, depending on the indication. Improvements in battery life have also allowed for the incorporation of additional software, with one company incorporating accelerometers in these devices. The stimulation programming can then detect changes to the patient’s body position and make adjustments accordingly. As more powerful and accurate pulse generators become available, they are now being paired with biofeedback systems for more accurate treatments.
Deep Brain Stimulation (DBS)
There are two different classifications of brain stimulation: deep brain and cortical. Although the exact mechanism is not fully understood, brain stimulation is thought to work by creating electrical interference that disrupts abnormal signals in the brain that can lead to tremors or dystonia. The electricity given to a particular area of the brain stimulates cells, which in turn can help with several conditions. The FDA approved DBS usage as a treatment for dystonia, essential tremor, medication-resistant epilepsy, Parkinson’s disease, and medication-resistant obsessive-compulsive disorder. Deep brain stimulation continues to face competition from the use of medication to treat Parkinson’s disease. However, the DBS market benefitted from the results of the Controlled Trial of Deep Brain Stimulation in Early Patients with Parkinson's Disease (EARLYSTIM) trial conducted by the University Hospital Schleswig-Holstein, which showed a benefit to the use of DBS for early-stage Parkinson’s disease.
Sacral Nerve Stimulation (SNS)
Sacral nerve stimulation devices are for the treatment of urge incontinence, urinary retention, fecal incontinence, and urinary frequency symptoms. The SNS is also used as a treatment for chronic constipation in some countries. It has also been shown to be effective in Parkinson’s patients with neurologic bladder symptoms. The device consists of electrodes that carry electric pulses from a generator to the patient’s sacral nerve. This acts to manipulate the behavior of the bladder, sphincter, and pelvic floor musculature. The generator is usually placed in the patient’s lower abdomen or buttock. SNS treatment is reversible, unlike some alternative treatment options and can be terminated when needed. The first stage of implantation includes an external stimulator usage for a period of three to 30 days. Following a positive evaluation, the device can be implanted.
Vagus Nerve Stimulation (VNS)
VNS therapy has been approved for over two decades for the treatment of epilepsy. Epilepsy is a neurological disorder involving recurrent unprovoked seizures, and patients who do not respond to treatment over long periods of time are considered to have refractory epilepsy. There are three standard treatment types for epilepsy: antiepileptic drug therapy, vagus nerve stimulation therapy, and surgery. The first-line treatment is drug therapy. If this is not effective, then VNS therapy is recommended. Surgery may be performed on some patients that are not well suited to vagus nerve stimulation therapy. Some patients who have refractory epilepsy use vagus nerve stimulation therapy as an adjunctive treatment to their medication. The FDA approved the use of vagus nerve stimulation for seizures in patients who are 4 years and older, have focal epilepsy, and have seizures that are not controlled with medications.
Additionally, there have been clinical trials observing the effectiveness of VNS for depression. The FDA approved the use of vagus nerve stimulation for the treatment of severe, recurrent unipolar and bipolar depression in 2005. To qualify for the treatment the person has to be older than 18, have chronic depression that is treatment-resistant, has not improved after trying four or more medications or electroconvulsive therapy, and continues standard depression treatments along with vagus nerve stimulation.
Gastric Electric Stimulation (GES)
Gastroparesis is a digestive disorder in which food moves through the stomach more slowly than normal because of partial stomach paralysis. The most common symptoms of gastroparesis include chronic nausea, vomiting, bloating, and difficulty eating. Numerous causes of gastroparesis include diabetes mellitus, anorexia, bulimia, lupus, and brain disorders and it is associated with increased mortality due to dehydration and weight loss. Gastroparesis’ first line of treatment usually includes dietary modifications and medications to control blood sugar level, in addition to medications affecting stomach muscle contractions. Candidates for gastric electrical stimulation include those who suffer gastroparesis but have not responded to conservative therapies, such as dietary adjustments and oral medications. In addition, patients who are unable to tolerate the medications used to control symptoms are often good candidates for GES.
Market Trends
The neuromodulation market is expected to experience high growth rates in the upcoming years, with the highest growth being observed in the Middle East and Africa, though North America and Western Europe currently represent the largest percentage of the market due to the high cost of neuromodulation devices.
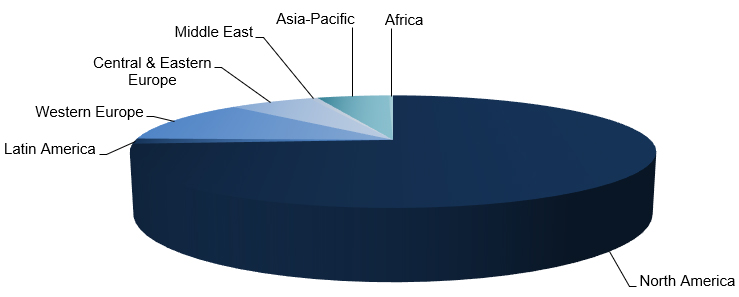
The sacral nerve stimulation devices segment is expected to have the highest growth rate, followed by the deep brain stimulation market. The increased use of rechargeable implants is a significant reason for the high growth rate in sacral nerve stimulation. Rechargeable generators are becoming more popular due to their lower long-term costs compared to non-rechargeable generators. Deep brain stimulation, on the other hand, is a very invasive treatment that requires generator replacements every three to five years. However, replacement procedures are less expensive and have lower risks of complications. The growing popularity of the Ativa SC and non-rechargeable systems by Abbott and Boston Scientific may increase the proportion of replacement procedures.
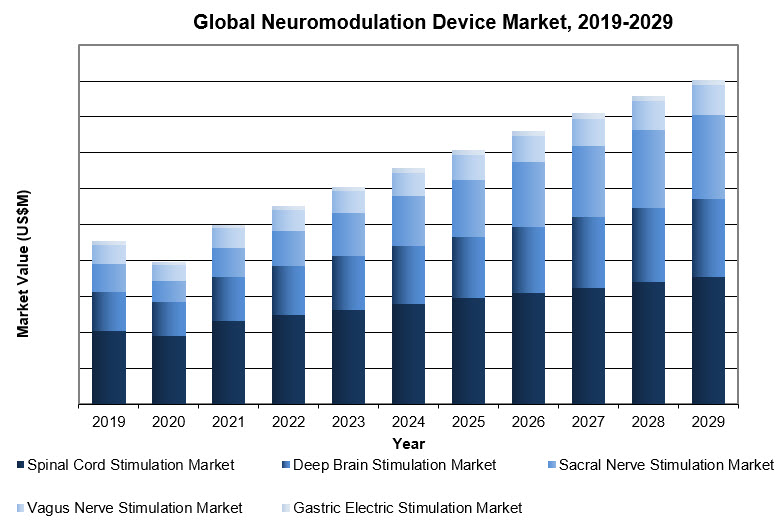
Medtronic is currently the leading company in the neuromodulation market worldwide, thanks to the popularity of its InterStim therapy system product line. Boston Scientific is the second-leading competitor in the market, followed by Abbott.
It is worth noting that Abbott has the smallest implantable pulse generator on the market, the BurstDRTM stimulation, which is a significant innovation in the industry. This device has the potential to improve patient outcomes, as it allows for targeted stimulation and reduces the likelihood of unwanted side effects.
The global neuromodulation market has been growing significantly, and it is expected to continue to grow as the demand for these devices increases.
References
- Velasco, F. (2000). Neuromodulation: An Overview. Archives of Medical Research, 31(3), 232–236. https://doi.org/10.1016/S0188-4409(00)00063-1
- iData Research Inc. Global Neurological Device Market – 2023. Published March 2023. Accessed April, 2023.
 About the Authors:
About the Authors:
Kateryna Hronska is a research analyst at iData Research. She works on research projects regarding the medical device industry, publishing the Global Neurological Device Market research report.
 Kamran Zamanian, Ph.D., is CEO and founding partner of iData Research. He has spent over 20 years working in the market research industry with a dedication to the study of medical devices used in the health of patients all over the globe.
Kamran Zamanian, Ph.D., is CEO and founding partner of iData Research. He has spent over 20 years working in the market research industry with a dedication to the study of medical devices used in the health of patients all over the globe.
