Anti-Choking Devices Could Provide Heimlich Maneuver Alternative
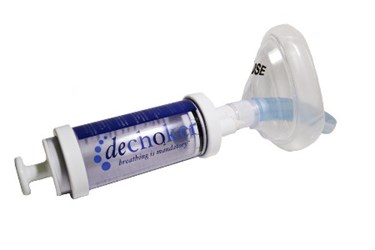
A medtech start-up in Louisville, Ky., has developed a device that uses simple physics to unblock clogged airways and potentially prevent choking deaths. Unlike the Heimlich maneuver, which has been known to cause various complications, the Dechoker device works in seconds, and if necessary, can be self-administered.
A U.S. National Safety Council report released in 2011 reported that choking causes 4,500 deaths per year among Americans — the fourth leading cause of unintentional injury deaths behind poisonings, motor vehicle crashes, and falls. While choking is a risk for people of all ages, children and the elderly are particularly susceptible.
A chocking victim has approximately seven minutes between obstruction and brain death. Since it was introduced in 1974, the Heimlich maneuver has saved thousands of lives because it can be administered quickly, while an ambulance is on the way. However, the procedure, which is almost always performed by amateurs, is not without significant risks.
In an article published in the Journal of Trauma Injury, Infection and Critical Care, medical experts reviewed the potential complications of the Heimlich maneuver, which include rib fractures, tissue perforation, and — in very rare cases — aortic valve cusp rupture. The authors advise anyone who has undergone the procedure seek follow-up care with their physician if symptomatic for these conditions.
In addition to potential complications, there are other limitations to the Heimlich maneuver: Another person must be available to provide assistance and that person must be strong enough to perform the procedure properly. Thus, the Dechoker was designed by Alan Carver, who was frustrated during a CPR class that there were no alternatives should the Heimlich maneuver fail or be impossible to perform.
The Dechoker uses a face mask that seals over the nose and mouth and is connected to a plunger system. Pulling the plunger back creates vacuum pressure that dislodges the obstruction. If the first try is unsuccessful, the air (or fluid) is vented through a valve when the plunger is depressed, allowing it to be pulled back again.
Carver told the Insider Louisville that he met with FDA consultants, doctors, and engineers before submitting the device to the FDA for approval. Christopher Kellogg, president of Dechoker, said that the company had to improvise on the Red Cross application because there was no category for anti-choking devices.
After five years of development, the Insider Louisville reports that the company’s FDA application was accepted six months ago, and the device is currently available for $150 per unit. The article also reports that the company has put aside 1,000 units to be given out for free to patients who have an elevated choking risk due to a medical condition, such as those with Cerebral Palsy, dementia, or other swallowing disorders.
After four months on the market, the device is receiving worldwide interest from foreign investors and competitive devices have also entered the market. LifeVac, a company that has designed a similar product, presented at the American College of Gastroenterology’s 2015 Annual Conference in Hawaii.
