APIC 2000: Needle-safety devices duel for product spotlight with antimicrobial hand sanitizers, part I
MINNEAPOLIS – Infection control professionals scouting out the latest needle-safety devices due to new state mandates or likely state requirements certainly had their legwork cut out for them here at the 2000 Annual Educational Conference and International Meeting of the Association for Professionals in Infection Control and Epidemiology Inc.
That's because roughly two dozen suppliers of various types of sharps devices sporting protective features all competed for the anticipated demand. Manufacturers touted safety syringes, scalpels, needles, needle-transfer systems and catheter securement systems. In fact, one company billed the APIC 2000 exhibition as its "coming out party" because it demonstrated prototypes that are six months away from market availability. Here's a roundup of who showed what.

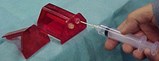
While many of the needle-safety device manufacturers promoted products designed with patient contact in mind, one company took a different approach. Cambridge (Rock Hill, SC) developed the Needle Barn StikStopper, which it designed to prevent accidental needlesticks during minor surgery and emergency laceration procedures.
"Most needle-safety products deal with manipulating needles in patients but don't address the needle-switching problem, particularly in the emergency department," Jerry Ballard, president, told Hospital Network.com. "That's where the major problem is." He further noted that these needlesticks typically go unreported. So Cambridge derived the Needle Barn StikStopper, particularly for nurses drawing and injecting lidocaine with traditional syringes. The product is especially applicable for the clinician who has to switch back and forth between the anesthetic drawing needle and the injection needle, according to Ballard. That clinician can do the required needle switching with one hand.
The Needle Barn StikStopper also sports a slot in the top of the device for the clinician to insert used suture needles following a procedure, effectively serving a dual purpose as a sharps container.

Sentry Syringe (Houston, TX) used the APIC exhibition to showcase its eponymous needle-safety device, which the company expects to release by year's end. The Sentry Syringe resembles a standard syringe equipped with a manual retraction mechanism. After the nurse administers an injection with the Sentry Syringe, she pulls back the plunger using a twist-and-pull motion, retracting the needle into the barrel of the syringe. When she hears an audible "click," the needle is secured, the plunger road is snapped off and she can dispose the device in an approved sharps container.
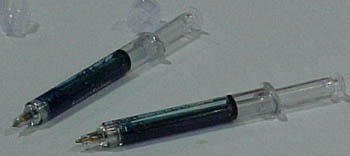
Kevin Fee, Sentry Syringe's director of product marketing and integration said his company's product accepts all standard luer needles and can be used for subcutaneous and intramuscular injection, as well as for intravenous applications using standard luer access ports.
SafeGard Medical Products (Woburn, MA) demonstrated several different needle-safety devices as part of its overall safety needle system, each with its own nuances. The SoloGard breakaway syringe includes just that feature: The piston end breaks away when the barrel is locked after delivery of the medication. SafeGard's NeedleGard device works with standard syringes by fitting needles with a protective plastic bar.
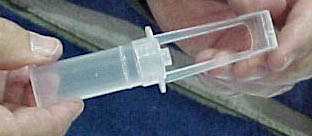
The company plans to release its new VectorGard needle-safety device in January 2001. VectorGard comprises a plastic hinged covering attached to a syringe's needle opening. The hinged device can be pulled back to serve as a stand and when the syringe is used on a patient and it can be closed to activate the needle-safety feature.
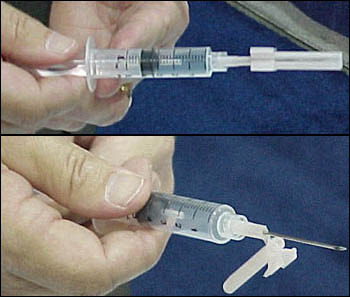
Safety Syringes Inc. (Carlsbad, CA) demonstrated its UltraSafe Needle Guards and Injection System, which are designed for pre-filled pharmaceutical glass syringes and cartridge systems. A nurse inserts the syringe into the UltraSafe Needle Guard device and snaps it into place. After the nurse administers an injection, she grasps the needle guard, sliding it forward until it completely covers the needle and "clicks" into place. Then the syringe can be disposed in a sharps container.

The maker of the Protectiv I.V. and Protectiv I.V. Plus safety catheter systems, Johnson & Johnson Medical Inc. (Arlington, TX), unveiled the latest needle-safety device added to its growing line. The new Accuvance I.V. safety catheter has a fully encased self-blunting needle. Lloyd Fishman, director of marketing for Johnson & Johnson Medical's Vascular Access area, said Accuvance currently awaits Food & Drug Administration 510(k) marketing clearance and should be available for purchase "soon."
Futura Medical Corp. (Solana Beach, CA) demonstrated its Lark line of disposable safety scalpels with retractable blades. The Lark features a user-activated locking inset to control whether the blade is exposed or hidden. Clinicians can extend and retract the blade single-handedly without worrying about an open sheath allowing the blade to peek out halfway. The Lark's size makes it convenient for inclusion in surgical and wound-care kits. The device is color-coded by blade size and features several styles of precision-sharpened blades and measuring scale.
SIMS Portex Inc. (Keene, NH) showcased its disposable Needle-Pro needle protection devices for arterial and venous blood draws. Clinicians can activate the Needle-Pro sheathing device by locking the device's hinged mechanism in place over the needle after it's used. They can do this with a one-step, one-handed downward push that clicks the sheath up into position.
C.R. Bard Inc.'s Bard Medical Division (Covington, GA) displayed its Bardex I.C. and Lubri-Sil I.C. Foley catheter systems. Both products sport a proprietary hydrogel and silver coating, which has been clinically proven to reduce the rate of urinary-tract infections (UTIs) in catheterized patients. Bard claimed that more than 300 hospitals are using its Bardex I.C. system and are seeing their nosocomial UTIs drop 80% compared to baseline data.

New Medical Technology Inc. (Zionsville, IN) demonstrated its NMT Safety Syringe, which officially hit the U.S. market in December 1998 after being marketed as Zero-Stik in Europe. By depressing the plunger, clinicians can make the needle in the NMT Safety Syringe automatically retract into the barrel, preventing a needlestick from occurring after an injection. Although the NMT Safety Syringe is currently available in a 3cc size with 21-, 23- and 25-gauge needles, the company indicated it plans to release additional sizes in the near future. Those additional sizes include a 1cc tuberculin and insulin syringe.
Venetec International (San Diego) highlighted its StatLock brand of catheter securement systems for peripheral intravenous, Foley, CVC, peripherally inserted central catheters (PICC) or midline catheters. StatLock securement systems include adhesive-backed disks with integrated catheter anchors to be attached to the skin and firmly hold a catheter in place. Among the StatLock products, the StatLock-IV, StatLock-IV Teddy, StatLock-IV Tandem, StatLock-IV Dual, StatLock-CV, StatLock-CV Teddy and StatLock-PICC are designed to eliminate unscheduled restarts and healthcare worker exposure to needlesticks. Venetec noted that its products have been shown to reduce overall catheter-related complications by up to 69%.
ICU Medical Inc. (San Clemente, CA) promoted its needleless Clave Connector product, which can be used as central and peripheral line and arterial line access devices, single and multi-dose vial adapters, IV bag access spikes and Y-site needleless adapters.
BD (Franklin Lakes, NJ) featured its popular SafetyGlide shielding hypodermic needle, which features "tiny needle" technology for such applications as insulin injections. SafetyGlide employs a shield that slides over the exposed needle after use. BD also highlighted its Insyte IV catheters, Insyte AutoGuard Shielded IV Catheters and its sibling winged model as well as its Saf-T-Intima Integrated IV Catheter Safety System.
Source: Hospital Network.com, sister Website to Medical Design Online.com
