Are You Compliant When It Comes To Pipetting?
Source: Sartorius
By Paulus Artimo, Joni Åke, Emilia Varhimo
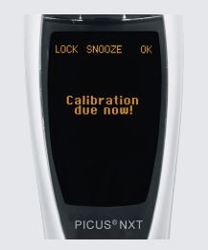
When developing or testing your medical device, there is always the question: are you following methods for current Good Laboratory Practice (cGLP) or current Good Manufacturing Practice (cGMP)? In this practical guide, we’ve compiled a list that you can follow to give you peace-of-mind on the topic of compliance. Download this guide to look into some tools and principles that can help with these demanding requirements, especially when it comes to your pipetting practices.
access the Application Note!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Med Device Online? Subscribe today.
Subscribe to Med Device Online
X
Subscribe to Med Device Online
