Are You Ready For The FDA's "Data Effect" Tsunami? 8 Steps To Prepare
By Matt Collins, CEO, Cignyl; and John Giantsidis, president, CyberActa, Inc.
The FDA is moving forward with its Data Modernization Action Plan (DMAP), the next leg of the Technology Modernization Action Plan (TMAP). Announced on March 3, 2021, DMAP is the agency’s overhaul of technology and data with the objective of bringing together increasingly disparate and diverse data sources to help understand and pinpoint emerging public health threats.
Modernization Action Plan (TMAP). Announced on March 3, 2021, DMAP is the agency’s overhaul of technology and data with the objective of bringing together increasingly disparate and diverse data sources to help understand and pinpoint emerging public health threats.
This sounds very noble, and using data as the basis of the FDA's regulatory decision-making seems to be an improvement. So, why would this be a tsunami for biotechnology, pharmaceutical, and medical device manufacturers? Do not misunderstand, data turned into knowledge improves understanding, decision-making, and, ultimately, outcomes. Businesses have learned this and are continuously improving their use of analytics as a competitive differentiator. However, what makes data valuable and informative can also be dangerous when wielded by a novice or used without proper verification and governance. On paper, the DMAP outlines several aspirational efforts to bring together a massive number of disparate data sources. What DMAP fails to address are the associated risks that come with predictive algorithms and poor modeling.
Predictive analytics and modeling are a form of artificial intelligence (AI). Predictive analytics uses machine learning to predict outcomes using historical data. Machine learning is an AI technology that finds patterns at scale with data sets. With machine learning, the models used to create predictions can act as black boxes. This means that how the predictions came to be is not fully understood. And while there are many instances of positive experiences with black-box algorithms, there are cautionary tales of algorithms gone bad. Let's be honest, the FDA does not always take an innocent until proven guilty approach with manufacturing firms.
The time is now for manufacturers to prepare themselves for the influx of questions, audits, observations, warning letters, and more with this new proclaimed approached to maintain data-driven regulatory decision-making.
Before we outline a road map to prepare for the incoming storm, we must discuss how social media will influence the FDA’s modernization of information. OpenFDA is an excellent source of FDA data accessible to the public (note the warning to avoid using this information in making medical decisions). The goal of providing data access to all puts manufacturers in the driver's seat to better control their destiny. What is missing? There are over a quintillion bytes of data generated daily with social media outlets.1 Most social media information (e.g., Twitter, TikTok, Instagram, reviews on Amazon, etc.) does not apply to manufacturers. However, there is a small percentage of this information that significantly impacts your business. Guess what? The FDA plans to monitor and assess this information under the umbrella of protecting public health. To prepare for this broad sweep of informational overload, manufacturers need to expand their post-market system capabilities by creating their own manufacturer Data Modernization Action Plan (mDMAP). Here’s the eight-step method to create your own mDMAP.
1. Walk The Walk With Predictive Analytics
Many manufacturers proclaim they have predictive capabilities, but instead they outline archaic approaches toward demonstrating state-of-the-art devices, made under state-of-the-art conditions, with state-of-the-art outcomes. Repetitive descriptive statistics only tell the history and remain a passive approach to post-market monitoring of signals. Descriptive analytics does not facilitate the much-needed dynamic monitoring. Without dynamic monitoring, manufacturers cannot proactively respond to information and prevent significant business disruption. The FDA is going predictive. Manufacturers need to prepare themselves to stay ahead of the curve. Instituting mDMAP can be a source of competitive advantage and significantly decrease the time spent writing fiction as to why your devices remain the best of the best.
2. Institute New Capabilities Into Your Post-market Surveillance Listening Systems
This will bolster your ability to respond to user experiences with your competitive devices (your opportunity to make quality a competitive advantage). As your mDMAP program evolves, your business vernacular will need to standardize around machine learning, predictive algorithms, and predictive intelligence. mDMAP is your way to get intimate with new data sources and new ways of seeing the information. Avoiding this intimacy increases your risk of "for cause" audits (we all know these audits go well!).
The bulk of new data is unstructured, which means the data is not conveniently in the form of a table with structured rows and columns. It is textual, video, pictures, etc. This lack of structure makes it difficult for the quality professional to understand, evaluate, and use it to support prevention. However, anything worth having is typically difficult at first. Unstructured data represents your new gold mine for post-market enlightenment. Anyone connected to the internet can voice their opinions. The ability to listen means manufacturers need to optimize their listening systems.
3. Use The A+B=C Formula
Given that we understand A (your current post-market listening system state) and C (the future post-market listening system state), we can solve for B (what you need to do to stay ahead). The same thing goes for our ability to transform our post-market listening systems and predictive analytics program. The first step of a great mDMAP approach is to outline your targeted future state (or variable C). To create a targeted future state, paint a picture of the destination (what you want to accomplish). Next, define your current state (or where you are currently sitting, variable A). Painting a realistic picture of the current state is difficult for many manufacturers, as it requires a lot of arduous self-reflection and realizations. What are your current capabilities, what are your current data sources, do you have a quality warehouse or data lake? By outlining the current post-market state and painting a picture of the destination, you effectively can solve for B – how to achieve your new mDMAP realities.
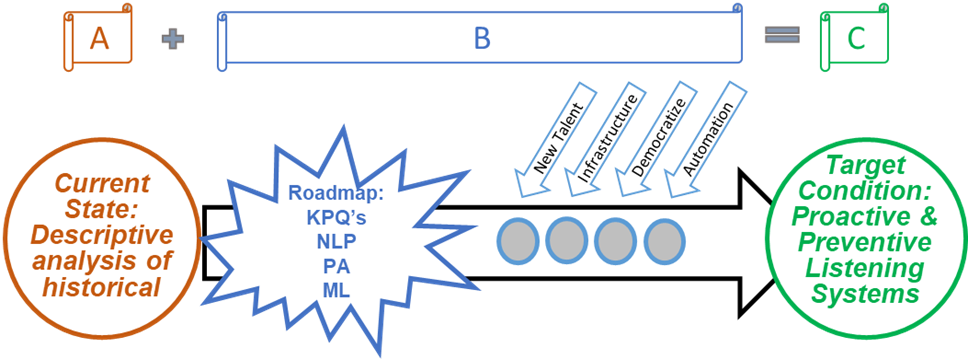
Figure 1. Road map to mDMAP
Variable B represents the road map from your current condition to the mighty target condition. Transforming your post-market listening systems and predictive capabilities requires investment in your infrastructure and some sweat equity. Before you begin building, the path to enlightened post-market and predictive systems begins with a cross-functional assessment of key performance questions (KPQs). Many people and businesses jump right to KPIs (key performance indicators), but the rocket fuel in any analytics program begins with KPQs.
4. Develop Your Key Performance Questions
Your ideal KPQs will allow you to develop a customized suite of information that delivers exactly what you need and avoid the traditional report on everything where you learn nothing. KPQs need to be open-ended (as they are questions) so that the team can determine:
- Which KPI answers the question?
- What type of data is necessary to create the KPI?
- Where does this data reside?
- Do we have access to such data?
Capturing the answers to these questions through a simple matrix helps to archive the knowledge, aligns the organization, and, most importantly, allows a new person to understand the intelligence behind the data analysis. You will be amazed at how powerful such a simple document becomes with immediate improvement to an existing post-market intelligence system. Having a prepared, yet simple and solid starting point, succinctly aligns the organization on your impending journey toward mDMAP bliss. Additionally, put this matrix in an existing procedure and be amazed at your ability to tame outside regulatory authorities.
5. Bring In New Skills And Capabilities (People Talent)
As people leave the organization or the business grows, the organization must focus on different skills and capabilities. As people exit the organization, we typically replace them with others with a similar skillset. Our mDMAP journey requires a more thoughtful approach to new employees. Use these opportunities to look for the skills needed to drive your future state. An excellent method to support skill requirements is to draw three circles and outline three high-level skills required. As an example:
- Circle 1 = Domain expertise (e.g., quality, regulatory, technical, etc.)
- Circle 2 = Computer hacking (e.g., programing skills such as Python, R, or data analysis skills with Power BI or Tableau)
- Circle 3 = Math and statistics (e.g., multivariate, uni and bivariate, etc.)
Once you have your three main topics, you can add subcategories within the circles. Why use three circles? It is easier to digest how to think about the future skills needed using such an approach. Jumping into a job description makes it easy to fall into the trap of using what currently exists (or simply plagiarizing some other company’s description from Indeed or another job site). Upon completion of the three circles, you intersect them for your own personal Venn diagram. At the center of the intersection is the unicorn you are looking to hire. Figure 2 provides an example of a Venn diagram for a data scientist. Do not allow this simple diagram to limit your thoughts on skills and capabilities. Three circles are not a limit but are a tool. Be thoughtful; as the number of circles grows, so will your difficulty in acquiring a unicorn. If you begin to find you require more than four circles, chances are you have two different jobs you need to fill.
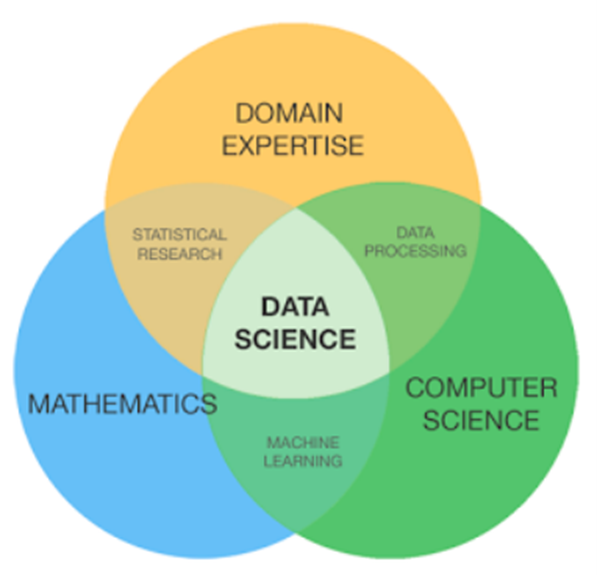
Figure 2. Venn diagram of a data science unicorn.
6. Leverage Existing Infrastructure Or Look Into New Infrastructure
By new infrastructure, I do not mean significant capital investment. Remember, this is a journey that will take multiple years. Initially, most companies are using an office suite (e.g., Microsoft, Google, Salesforce, etc.) that they can leverage. If your company currently uses Microsoft, PowerBI is a simple tool that integrates easily for a minimal monthly fee. Don't get caught in the big, expensive solution. Start simple, and as the company begins to reap the benefits of their initial investment, evolution will continue.
How can this be accomplished? First, leadership must break the barriers on access to information and data. Many organizations create significant barriers to accessing data. IT must become a shepherd for sharing data and access. This does not mean being irresponsible with security; it means training and facilitating read-only access to data sources (one-way streets to pull but not write information) and creating data warehouses and data lakes that can be accessed by all (real-time financial information notwithstanding, some information must stay protected to avoid insider trading). Through a combination of access and training using free sources and in-house experts, learning and institutional knowledge will begin to blossom.
7. Democratize Your Information
Democratization of your information – driving the training and tech into all organizational ranks – requires a lot of focus and effort. Through democratization, the exponential growth of knowledge and improvement will rise like a phoenix. There are plenty of free tools available on the web or at edx.org to help with this effort. Heavy dollar investment is not required, only heavy investment in your sweat equity.
Democratization serves two significant purposes. It grows the institutional knowledge and keeps your workforce learning. A business can only grow if its people are growing (learning is the foundation of people growth). Democratization is a form of visual cues that provides a strong and robust safety net capable of seeing information and issues previously unnoticed. Think of it like this: Would fans attend a baseball game if they could not see the score? By driving the information into all ranks, the score is known by all and, more importantly, quality is seen as more than a function; it becomes transformed into an institutional capability embraced by all.
8. Automate – But Not Yet
Many companies want to jump to automation. Avoid this urge. Technology accelerators have their place; however, technology-induced change without proper process or relevancy is a program killer and money pit all in one. People become enamored with the flashing lights and new technological toys. Smart businesses have learned to run, but you must first walk, and to walk you must crawl. The reality is that focusing on finding the right future talent and initial infrastructure and democratizing your information will serve to move your program from a crawl to a walk. Once these elements are going well, you can begin the next phase of leveraging better prediction through automation and machine learning.
Now that you have the right talent, a simple to understand infrastructure, and a well-versed organization, you are primed to use technology to help accelerate good processes and begin to converge with more expansive data sets. Applying the right set of machine learning tools allows a business to create a more informed analysis of existing data for deeper insights and statistical patterns.
Our historical data sets can be used to train algorithms to understand behavioral patterns, anticipate problems, and effectively allow for timely and prepared action. Additionally, these learning algorithms can actively monitor the many different disparate data sources that generate new information daily. Doing so will facilitate the development of early warning signals or, better yet, outline things that are going well. Understanding what works well allows a business to exploit a competitive advantage within its quality program.
Another benefit is that these predictive algorithms can be used to monitor industry trends, including with regulatory agencies and competitive businesses. This monitoring effectively helps you see the winds of change and stay away from oncoming obstacles.
An effective method to determine your automation road map is to evaluate what is working well; this is a candidate to automate and evolve through machine learning. Start with a new set of KPQs and create three circles for your automation Venn diagram. Each circle wraps around an automation concept. For example, what drives customer behaviors around the quality of your products, what issues are your customers passionate about, what type of data exists, and what types of algorithms can be used to explore the data? Combine the three circles into your Venn diagram. The overlap will help shine the spotlight on how to attack and automate your next steps toward an automated predictive program.
Conclusion
The FDA's initiative could have significant and costly ramifications for manufacturers, whether in biotechnology, digital health, pharmaceuticals, and anywhere in between. However, by following mDMAP we can mitigate the likelihood of regulatory scrutiny. Starting your mDMAP integration voyage now allows you to stay ahead of the data management curve and protects your business. More importantly, your mDMAP journey will provide greater understanding and help you proactively pivot using the voice of your customers. The proactive capability drives your ultimate goal of doing well by doing good. Or, said another way, we ensure safe and effective outcomes through measured and consistent approaches.
Reference
- Vuleta, B. (2021, January 28). How much data is created every Day? [27 POWERFUL Stats]. Retrieved April 02, 2021, from https://seedscientific.com/how-much-data-is-created-every-day/#:~:text=Every%20day%2C%20we%20create%20roughly%202.5%20quintillion%20bytes%20of%20data
About The Authors:
 Matt Collins is a cofounder of Cignyl and serves as its CEO. He brings over 25 years of industry experience with a track record for building consistent and reliable outcomes. He has driven critical turnarounds for complex situations such as warning letters, consent decrees, international regulatory problems, implementation of global systems, network optimizations, and mature business situations. He holds a doctorate in business administration with an emphasis in healthcare and leadership from California Intercontinental University, an MBA from Marquette University, and a BS in molecular biology from the University of Wisconsin Parkside. He can be reached at matthew.collins@cignyl.com.
Matt Collins is a cofounder of Cignyl and serves as its CEO. He brings over 25 years of industry experience with a track record for building consistent and reliable outcomes. He has driven critical turnarounds for complex situations such as warning letters, consent decrees, international regulatory problems, implementation of global systems, network optimizations, and mature business situations. He holds a doctorate in business administration with an emphasis in healthcare and leadership from California Intercontinental University, an MBA from Marquette University, and a BS in molecular biology from the University of Wisconsin Parkside. He can be reached at matthew.collins@cignyl.com.
 John Giantsidis is the president of CyberActa, Inc, a boutique consultancy empowering medical device, digital health, and pharmaceutical companies in their cybersecurity, privacy, data integrity, risk, SaMD regulatory compliance, and commercialization endeavors. He is also a member of the Florida Bar’s Committee on Technology and a Cyber Aux with the U.S. Marine Corps. He holds a Bachelor of Science degree from Clark University, a Juris Doctor from the University of New Hampshire, and a Master of Engineering in cybersecurity policy and compliance from The George Washington University. He can be reached at john.giantsidis@cyberacta.com.
John Giantsidis is the president of CyberActa, Inc, a boutique consultancy empowering medical device, digital health, and pharmaceutical companies in their cybersecurity, privacy, data integrity, risk, SaMD regulatory compliance, and commercialization endeavors. He is also a member of the Florida Bar’s Committee on Technology and a Cyber Aux with the U.S. Marine Corps. He holds a Bachelor of Science degree from Clark University, a Juris Doctor from the University of New Hampshire, and a Master of Engineering in cybersecurity policy and compliance from The George Washington University. He can be reached at john.giantsidis@cyberacta.com.
