Are You Satisfied With The FDA?

By Bob Marshall, Chief Editor, Med Device Online

I attended MedCon 2017 at Xavier University in beautiful Cincinnati, Ohio (actually, it rained nearly the entire time). Regardless of the weather, MedCon was excellent. I heard about trends from industry leaders and regulators, I met interesting people, and I gathered some great ideas for topics that I’ll be sharing with you in coming weeks.
One bit of information that captured my attention stemmed from an Update from the Office of Device Evaluation by Angela Krueger, FDA’s acting deputy director for engineering and science review. Amid Krueger’s presentation of CDRH’s current performance metrics and future performance goals under MDUFA IV, she talked briefly about the customer satisfaction rating for CDRH. Wait… what? CDRH has a customer satisfaction metric? I was immediately curious, and I was ultimately surprised by what I learned...
As I think about the baseline for customer satisfaction, I can almost hear Keith Richards’ fundamental 1965 guitar riff that became the basis for the Rolling Stones’ (I can’t get no) Satisfaction. There certainly are people who seem unable to give the American public much satisfaction these days: Gallup polls indicate that only 20 percent of U.S. adults approve of Congress’ recent work; 80 percent of those polled “can’t get no satisfaction” from the performance of their Senators and Representatives. Turning to the White House, Gallup also reports that about 40 percent of Americans approve of the job being done by U.S. President Donald Trump. This is significantly better than Congress, but well below the average for all U.S. presidents since 1938, which averages 53 percent. Given these poor results for the legislative and executive branches of the U.S. government, one might expect the FDA to follow suit and fall short. Well, hold that thought…
Don’t Worry, Be Happy!
What does it take to satisfy us? I turned to industry benchmarks provided by the American Customer Satisfaction Index for the calendar year 2016. First, I looked for things we use every day: for cellular telephones, Apple set the highest benchmark with a customer satisfaction index of 81 percent. Google held the top spot for internet search engines, 84 percent. Internet retail was dominated by Amazon with a customer satisfaction index of 86 percent. And, since all of this reading may be making you hungry (or maybe thinking about being at a Rolling Stones concert gave you the munchies), Trader Joe’s was the top-rated supermarket, with a customer satisfaction index of 86 percent.
Looking at the level of satisfaction we have with these generally well-respected brands, we get an idea of what we might expect from customer service in the commercial world. So, are we satisfied with the FDA?
Customer Satisfaction With FDA CDRH
The results of the FDA CDRH customer satisfaction survey for the period in progress (1/1/2017 to 6/30/2017) show 88 percent satisfaction as of May 8, 2017 at 1:45 pm EDT.
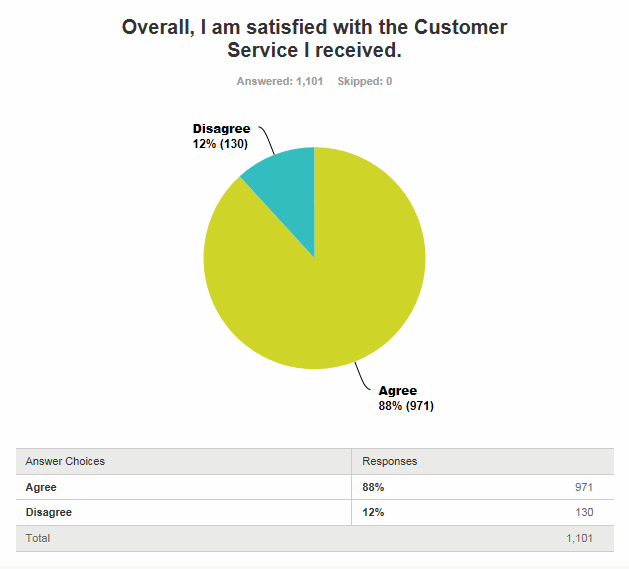
Fig. 1 — FDA CDRH Customer Service Satisfaction.
Source: https://www.surveymonkey.com/results/SM-QDY3SJ5F/
To add some historical perspective, here are the five most recent customer satisfaction scores for CDRH measured over six-month periods:
- June 16, 2014 - December 31, 2014 — 83 percent
- January 1, 2015 - June 30, 2015 — 88 percent
- July 1, 2015 - December 31, 2015 — 87 percent
- January 1, 2016 - June 30, 2016 — 84 percent
- July 1, 2016 - December 31, 2016 — 85 percent
CDRH has consistently been receiving customer satisfaction scores near the 85th percentile, meaning medical device manufacturers have regularly given the FDA’s CDRH a solid “B” grade. This surprised me, as I expected that more people would be dissatisfied with the FDA’s customer experience. I have frequently heard industry professionals complain about the lack of experience among some FDA staff with whom they have interacted. I have seen request for additional information (AI) letters, penned by the FDA in response to a 510(k) submission, asking for data that already was included in the original submission package. I also have had a handful of pre-submissions experiences where the FDA’s written response to the pre-sub package arrived just hours before the scheduled review phone call, allowing essentially no time for the med device company to review and digest the information. Perhaps my experiences are rare (it is a very small sample set, compared to industry at large). Or, maybe there is a silent majority of industry professionals who genuinely are satisfied with the service they have received from CDRH.
It is a difficult dynamic because, even though CDRH is soliciting customer feedback, they are part of a regulatory enforcement agency. Sure, your feedback is anonymous, but do people believe that their “constructive” feedback will lead to any substantive changes? I hope that we in the medical device industry take every opportunity to provide honest and productive feedback to CDRH, via this survey or other available means. The MDUFA initiatives are designed to improve the FDA’s responsiveness, enabling the medical device industry to more efficiently and predictably create safe and effective products.
I am curious to hear your reaction to this data. What grade would you give CDRH, based on your interactions with the agency, and why? Let us know in the Comments section below.
