Assuring Reliability In Medical Device Manufacturing Using Automation And A Digital Factory
By Ravi Subrahmanyan and Juergen Lindner
When medical electronics fail, patient outcomes may be significantly impacted. To avoid this dilemma, medical electronics are specifically designed to be highly reliable. Unfortunately, that approach alone is no longer sufficient given the increasing miniaturization and functional integration of medical electronic assemblies, lack of redundancy, faster product lifecycles, and use of new combinational technologies. This article details how a controlled, data-rich, and cost-efficient environment is being used by Micro Systems Engineering, Inc. (MSEI) to deploy a fail-safe, fast response strategy for manufacturing highly reliable medical electronics. This innovative data-rich approach allows companies to be assured that their products are manufactured consistently, and that any anomalies in performance are rapidly detected and responded to accordingly.
Failure of medical electronics can impact patient outcomes, either through improper diagnostics or insufficient therapy. Often, reliability of such assemblies is assured by component and process design margins. However, given the increasing miniaturization and functional integration of medical electronic assemblies, faster product lifecycles, and use of new combinational technologies, a controlled, data-rich, and cost-efficient environment has become increasingly relevant.
Micro Systems Engineering (MSEI), a subsidiary of Micro Systems Technologies (MST), has deployed a fail-safe, fast response strategy for manufacturing highly reliable medical electronics. The strategy relies on scalable factory automation and a digital factory concept to eliminate manual handling of product assemblies and data, automate workflow, and preserve traceability information, while making the manufacturing process much more efficient and future-proof. The result is an innovative and unique data-rich approach that allows companies operating in industries demanding high reliability to be assured their products are manufactured consistently, and that any performance anomalies will be rapidly detected and responded to accordingly.
Challenges Facing the Medical Device Industry
The medical device industry today faces many challenges that are driving the need for a new approach to manufacturing reliable medical devices. Miniaturized microelectronics often requires advanced assembly technologies despite the low tolerance to manufacturing quality risk. At the same time, prognostic reliability assurance is often not practical in early start-up, low unit volume environments with such unique designs.
The need for reduced development cycle times, higher efficiency, and lower cost may at times require optimization of validation scope, test, and quality assurance efforts. When unit volumes are relatively low, the supply base leverage is limited. Access barriers to the latest technology, as well as regulatory lead times, can pace the deployment of cost-optimized solutions. Additionally, the rapidly changing global, political, and economic environment often demands a manufacturing strategy that is independent of low-cost labor. As a result of these challenges, a scalable and efficient manufacturing environment is required - one that meets the miniaturization, reliability, and cost trends for medical electronics manufacturing.
Modern Automation and the Digital Factory
An ideal solution to these medical electronics manufacturing challenges is to deploy a “product agnostic,” tightly coupled physical and digital factory that allows “no touch” product and data handling, along with a traceable, automated workflow (Figure 1).

Figure 1. A look at manufacturing automation, past and present, details an evolution from a manual, paper-driven process to one defined by machines and digital file storage and analysis.
The physical factory refers to the actual equipment, tooling, and aids for manufacturing on the factory floor. Physical factory automation enforces workmanship standards by avoiding anomalous handling, and imposing both strict recipe management and tight process control. Automated test and inspection methods can provide repeatable, quality relevant data. Consequently, an automated physical factory can proactively address product quality and improve dependency on direct labor.
The digital factory is a companion virtual representation of the physical factory. It contains the “know how” to manufacture a product, as well as the quality and transactional data that is unique to each serial number manufactured. The digital factory identifies the right workflow and recipes for that serial number and communicates this information to the physical factory. As the product moves through the physical factory, the digital factory can dynamically prescribe manufacturing operations and validate recipes and inspection parameters. Manufacturing errors are avoided by physical and digital interlocks.
During or after the manufacture of a product, the digital factory enables rapid response to quality resolutions. Paper-based traceability often requires analysis of data by transcribing and analyzing it in a spreadsheet. Extracting even the most basic data for containment of an affected product may take hours. With the digital factory, that same task is often automated or can be performed with just the click of a button. As a result, indirect staff members are utilized more for their knowledge and specialized expertise, rather than the mechanics of manually extracting and analyzing data. Manufacturing problems are therefore found and resolved quicker.
By reducing the dependency on labor, direct or indirect, physical and digital factory automation also help assure quality, speed manufacturing, and drive down costs. While the physical factory automation facilitates scalable manufacturing, relatively independent of direct labor, digital factory automation can help simplify and improve product traceability, change control, and process management. This makes it easier for manufacturers to meet the requirements of regulatory bodies around the world.
A New Strategy for Manufacturing Reliable Medical Electronics
MSEI has developed a fail-safe fast-response strategy for assuring the development and manufacture of highly reliable medical electronics that relies on miniaturized product designs, as well as scalable physical and digital factory automation. At its core, this strategy involves platform processes such as surface mount technology, automated test and visual inspection, and automated final assembly. Platform component technologies such as high-density interconnects based on MST (DYCONEX) flexible substrates, MST (MSE - Micro Systems Engineering GmbH) area array packages, and standardized active and passive discrete components and sensors, are integrated using these platform processes.
MSEI’s strategy utilizes off-the-shelf and custom “smart machines”, to allow validated processes, material handling and digital connectivity within and between machines (Figure 2). A paperless “smart factory” enforces the use of released recipes, automated set-ups, prescriptive workflows, and controlled material movement. Scalable and failure-tolerant network, storage, and security systems enable this smart factory. An interconnected Enterprise Resource Planning (ERP) system (SAP) manages configurations, materials, work-order routing, and planning. A Manufacturing Execution System (MES, Siemens-Camstar) ensures the execution of prescribed workflows and collects transactional and quality data for each product by serial number. Standardized and ad hoc reports are used to monitor key factory metrics and for early warnings and rapid problem resolutions (Figure 3).
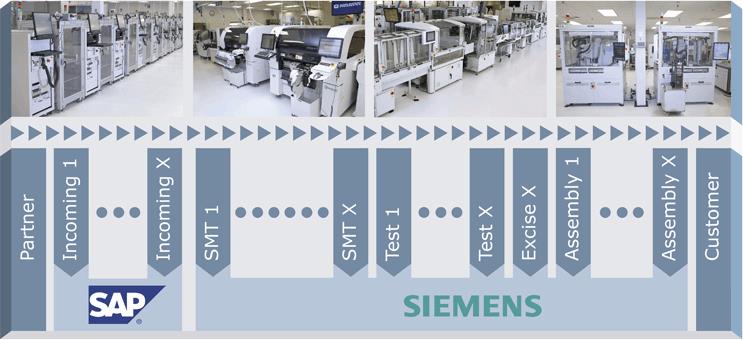
Figure 2. MSEI’s interconnected factory comprises physical factory automation (on the top) and digital factory automation (on the bottom). The manufacturing process flow is also highlighted.
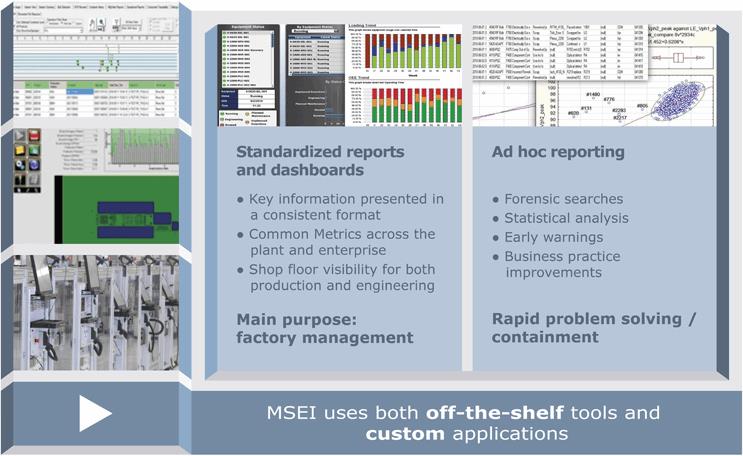
Figure 3. MSEI’s manufacturing strategy employs a range of custom and off-the-shelf business intelligence in a data-rich environment. Doing so provides traceability and allows it to quickly find and take care of performance anomalies.
The smart machines, factory, and enterprise infrastructure are used in development, prototyping, and high-volume manufacturing, thereby allowing seamless product lifecycle transitions and ubiquitous data availability. Customizable, automated and traceable interlocks in the physical and digital factory control the quality of each unique serial number. Because there are no separate development and manufacturing systems, feedback is rapid and “know how” is transferable through multiple lifecycles.
Uniquely Suited to Assuring Medical Device Reliability
MSEI’s proven manufacturing strategy was developed through over 17 years of physical and digital factory automation efforts. As a result, MSEI’s manufacturing plant output has risen five times compared to year 2000, while its head count remains the same (Figure 4). During that time, MSEI has also increased its ability to efficiently serve multiple customers while being an integrated part of a connected, international manufacturing network. At the end of the day, those capabilities, built on MSEI’s unique and innovative data-rich manufacturing strategy, provide customers with the assurance that their medical devices will be consistently manufactured with the highest level of reliability, every time.
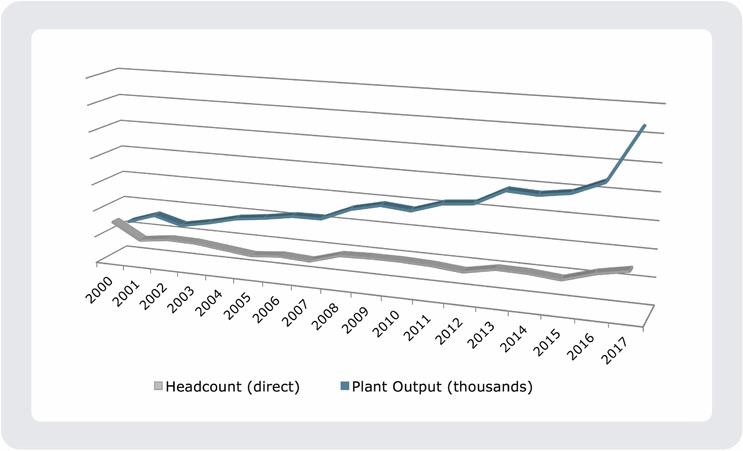
Figure 4. MSEI’s digital and physical factory automation has reaped tremendous rewards in terms of increased plant output, with little to no increase in head count.
About the Authors
Dr. Ravi Subrahmanyan is the Executive Director, Advanced Technologies Group, Micro Systems Engineering, Inc. (Lake Oswego, OR). He leads a team for selection and deployment of design and manufacturing technologies for use in high-reliability microelectronics applications.
Juergen Lindner is the Co-President and General Manager of Micro Systems Engineering, Inc. (Lake Oswego, OR). He leads the development and manufacturing groups that provide supply chain, development and manufacturing solutions for high reliability medical microelectronics applications.
