Can Better Diagnostics Cure Our Healthcare System?

By Bob Marshall, Chief Editor
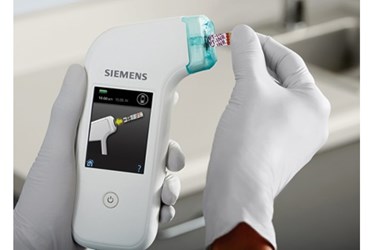
Perhaps no area of healthcare is experiencing more dynamic growth and providing more value for the money than diagnostic testing. According to an EvaluateMedTech report from last year, annual world-wide sales of in vitro diagnostics (IVDs) are projected to swell to over $70 billion by the year 2022. Over the same time period, IVDs are also projected to retain their lead position with the largest worldwide market share of all device categories, 13.4 percent.
Will IVDs be able to fulfill this promise? They do have a distinct advantage in the value they provide. A survey of doctors in 2016 found that IVD testing guides approximately 66 percent of their clinical decisions; yet IVD spending is low, accounting for only 2.3 percent of overall healthcare expense in the United States. The old adage is true – early diagnosis and treatment is much less expensive.
The above information, as well as the partnership of Siemens Healthineers and point-of-care (POC) connectivity platform-maker Relaymed — announced earlier this year — led me to contact Michael Sampson, Senior VP, Point of Care Diagnostics, North America, Siemens Healthineers. Siemens Healthineers has a prominent presence in the diagnostic market, especially those used at the POC. Sampson, who boasts well over a decade of leadership experience in this space, shared some of his thoughts.
Plop, Plop, Fizz, Fizz… Wonder What My Glucose Is?
At times, it is hard to believe how far we’ve come. Sampson reflected on the humble origins of diagnostic testing: “75 years ago, there were only a few diagnostic tests. A simple thing like testing a urine specimen for diabetes was accomplished by dropping something resembling an Alka-Seltzer tablet in the sample and looking for a color change as it was fizzing.”
Sampson also mentioned the long history of arterial blood gas analyzers, which began in 1957 when John Severinghaus developed the first blood gas analyzer, which measured pH, PCO2, and PO2. In the mid-1960s, these units became commercially available and were used primarily in clinical laboratories.
From the very beginning diagnostic testing has been differentiated by location. When sample preparation was simple, reagents could be easily handled and measured, and the test could be safely performed at a doctor’s office or hospital, it was typically conducted at the POC. When the converse was true, or if the diagnostic test involved large, expensive equipment, clinical laboratories were the right choice. This also brought economics and the cost of conducting these tests into consideration. As fluidics, sensors, and electronics improved in the latter part of the 20th century, and analyzers became smaller and easier to use, these lines became further blurred. Diagnostic tests previously conducted only in clinical laboratories gradually began to migrate into POC environments. Finally, medical professionals and the healthcare industry began to ask the question, “where would it be better to perform these tests: in a centralized laboratory or at the POC?”
The answer depends on your priorities, the available technology, and ultimately, on the needs of the patient.
Just Because We Can, Doesn’t Mean We Should…
There are certainly competing priorities within the diagnostic space. We live in a society increasingly focused on convenience. I can log on to the local grocery chain’s website, select a bunch of groceries, pay for them online, and then drive over to the store, where employees will bring the order to my car and put it in the trunk. That’s crazy! (I know – soon, a drone will deliver them to my door step.) But for diagnostic testing, convenience alone is not enough. We also must have accuracy. If the grocery store forgets to include a can of peas that was ordered, we will have to improvise a meal. But if the Strep test for your sick child produces a false negative, he or she will not receive the appropriate treatment and may become gravely ill.
This is why my discussion with Michael Sampson was so interesting. His company covers the entire clinical pathway, from laboratory automation systems to molecular diagnostics and POC diagnostics, including the integration of information technology. Per Sampson, Siemens Healthineers’ goal is “a lab without limits.”
I asked Sampson about our cultural shift toward convenience — specifically, whether we would see a complete shift toward POC diagnostics, due to the convenience they provide. Sampson replied, “No. The central laboratory does not go away. We will continue to see coexistence between the central lab and POC diagnostics. Tests that are widely used, with results that are not time critical, will continue to be performed by the centralized lab. It just makes better economic sense.”
He went on to address some of the financial considerations of providing the most balanced overall solution: “70 percent of healthcare costs stem from managing chronic disease. Diagnostic testing creates the majority of its value through the ability to monitor chronic disease and enable us to keep it under control.”
So, if a large percentage of healthcare costs are related to managing chronic disease, and many of those conditions are managed with diagnostic tests through a central lab to minimize cost, what is the sweet spot for POC diagnostics?
“POC diagnostics make the most sense when time is of the essence. In the ICU, medical professionals are dealing with acute symptoms and need information rapidly to respond,” Sampson explained. In this situation, cost and efficiency are trumped by the need to save lives, as long as the POC diagnostic provides the necessary accuracy. The ultimate goal, Sampson continued, would be to provide lab quality results at the POC. This would reduce the decision to simple logic: Is the patient’s condition such that we need to run the POC diagnostic test right here, right now, to obtain immediate results? Or, should we send a sample from the patient to the central lab, wait for them to run the test, and receive the results in a day or two? It is about delivering efficient testing where it provides the highest value for the patient.
I imagine there are those who would always want the results sooner, rather than later (these probably are the same people who no longer want to follow me around the grocery store with my buggy – Yeah, I’m an excellent driver). However, in trying to manage healthcare costs, I do not think Medicare and private insurance would support all-POC testing, all the time, unless POC diagnostics can provide the same level of accuracy as central lab testing at the same, or lower, price point.
Alternatively, individuals with the means to do so could choose to pay privately for quicker results. It would be just like paying the additional cost for same-day shipping on your latest treasure from Amazon.
Hmmm… perhaps the next step in “personalized” medicine?
