Clinical Study Of Heart Assist Device Suspended After Patient Deaths
In accordance with FDA regulations, Sunshine Heart has suspended a clinical trial of its C-Pulse heart assist system following the death of four study participants. Though the company claims the deaths are not a result of the device, Sunshine Heart plans to submit a report to the FDA outlining their plans to update the study.
Sunshine Heart reported in a press release that, under the directive of the FDA, it has until March 16 to submit an IDE supplement explaining the reasons for the suspension and the company’s plans to resume the study. The FDA has 30 days to review their findings.
The FDA stipulates that a clinical trial must be suspended if more than three participants die while the trial is underway, and Sunshine Heart is acting in accordance with these rules. According to the company’s statement, a Clinical Events Committee has already ruled that two of the four reported deaths were not related to the implanted device, and the company expects similar ruling for the other two patients.
“We are confident this matter will be resolved in a very short time frame. While the current data suggests these incidents are non-device related, we have decided that in the absolute interest of patient safety, having a temporary pause in enrollment is the right course of action while we work with the FDA to discuss the findings,” said Dave Rosa, Sunshine Heart CEO.
Rosa continued by saying that public interest in the study continues to grow and enrollment for the study would continue while Sunshine Heart worked to resolve any possible issues concerning patient safety.
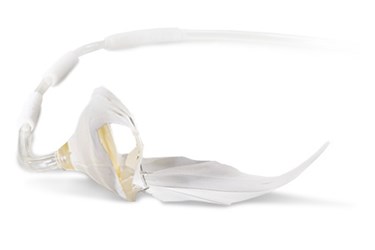
The COUNTER HF study is evaluating the safety and efficacy of the C-Pulse ventricle assist device, which was designed using balloon counter-pulsation technology to treat clinical symptoms associated with Class III and IV heart failure by decreasing the workload of the left ventricle and increasing blood flow to the coronary arteries.
The company is working under an investigational device exemption (IDE) from the FDA, which clears C-Pulse for clinical study. The device is CE marked for commercialization in Europe.
The company reports that trial enrollment has jumped from 7 to 100 in the first quarter of 2015. Sunshine Heart’s entry on ClinicalTrials.gov indicates an expected enrollment of 388 at over 40 clinical sites and a completion date in April 2017.
Sunshine Heart announced that it will host a conference call on March 17 following its submission of the supplement document to the FDA. Bill Abraham, a principal investigator on COUNTER HF, will be available to take questions.
Image credit: Sunshine Heart
