Conducting A Medical Device Stability Study: A Practical Guide
By Venkatesan Muthukrishnan, Johnson & Johnson

Medical devices must deliver consistent performance, safety, and quality throughout their claimed shelf life. To demonstrate this, manufacturers conduct stability studies — structured evaluations that measure how devices respond to environmental stresses such as temperature, humidity, light, and time. These studies underpin regulatory submissions, labeling claims, and quality assurance systems.
Although medical devices differ from pharmaceuticals, many stability principles align with globally recognized standards such as ICH Q1A-Q1F and WHO stability guidelines. Incorporating photostability assessments when products include light‑sensitive components ensures comprehensive stability characterization. (See: ICH Q1A(R2) Stability Testing of New Drug Substances and Products.)
This article walks through the purpose and process of stability studies in a clear, systematic way.
1. Why Initiate A Product Stability Study?
A product stability study is a scientifically justified investigation to confirm that a medical device maintains its functional and material integrity over its intended shelf life time under real‑world or recommended storage conditions. Common reasons to initiate a stability study include:
- New Product Development (NPD): to demonstrate that a newly designed device meets quality expectations during its intended shelf life.
- Life Cycle Engineering Changes: to verify that design updates, new materials, or manufacturing changes do not negatively impact stability.
- Research and Risk Assessment: to understand degradation pathways and failure risks or to support future regulatory filings.
In every case, the study must generate reliable data demonstrating that quality attributes remain within specification throughout the proposed storage period.
2. Planning And Designing A Stability Study
Effective stability studies begin with a carefully designed study plan.
Stability programs may combine multiple study types depending on the study intent, shelf life determination goals, and device characteristics:
- Real‑Time (Long‑Term) Stability: Stability samples are stored in chambers under expected normal temperature and relative humidity (RH) setpoint conditions (e.g., 30 degrees C/65% RH) for the entire intended shelf life duration (e.g., two to five years).
- Accelerated Stability: Devices are exposed to elevated conditions (e.g., 40 degrees C/75% RH) to predict long‑term behavior in a shorter period.
- Excursion/Stress Cycling: Cycles between extreme temperatures (e.g., minus 20 degrees C to 60 degrees C) to simulate transport and handling stresses that devices might experience in distribution.
- Photostability (Light Exposure) Testing: This is especially relevant when devices contain light‑sensitive materials or coatings. Per ICH Q1B, light exposure studies help determine whether product performance or materials degrade when subjected to controlled UV/cool white light stress conditions — ensuring labeling and packaging mitigate photodegradation risks. (See: ICH Q1B Photostability Testing.)
Combining these study types provides a comprehensive picture of how a device will behave over time and under environmental stress.
Developing Protocols
A written test protocol serves as the study blueprint. It should describe:
- objectives and justification of conditions
- storage conditions and durations
- sampling intervals
- details of types of testing and sample size at each pull and acceptance criteria
- test data collection, analysis plans, and reporting procedures.
Study Setup and Sample Logistics
Before the study begins:
- Configure the stability study in a laboratory information management system (LIMS) for sample inventory information, tracking, data capture, and audit control.
- Order stability test samples from manufacturing so that they reflect production specifications and representative lot characteristics.
3. Sample Conditioning And Storage
Once the stability team receives the medical device product test samples from manufacturing, the samples are verified, labeled, preconditioned, and stored under defined environmental settings in chambers per the study protocols. While medical devices aren’t pharmaceuticals, the framework from ICH Q1A(R2) and ICH Q1B provide well-established sets of storage conditions that can be adapted — particularly when device components (e.g., adhesives, polymers, reagents, sensors, or optics) might be sensitive to temperature, humidity, or light.
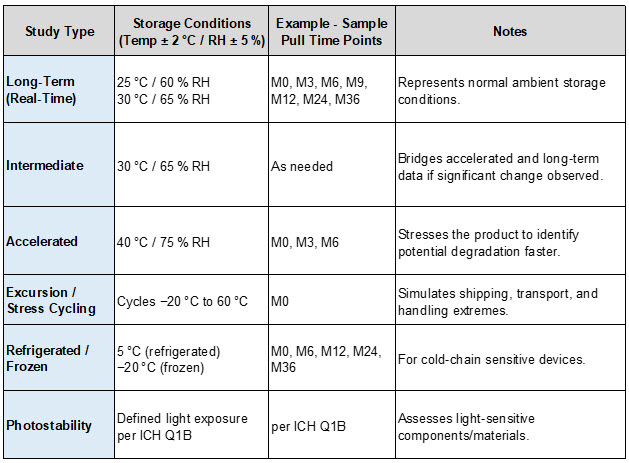
Table 1: Key Storage Conditions per ICH Q1A(R2) and ICH Q1B
4. Scheduled Sample Pulls For Testing
Key stability data comes from periodic sample retrievals (pulls) from the chambers according to the study design in the protocol; for example: Month 0, Month 3, Month 6, Month 9, Month 12, Month 24, Month 36 (or points tied to claimed shelf life).
At each interval:
- Pull designated sample quantities from controlled storage, per protocol.
- Then, hand over samples to appropriate testing laboratories.
Regular pulls build a chronological data set to detect trends such as gradual degradation, sudden failures, or stability inflection points.
5. Testing And Report Generation
Pulled samples are evaluated by specialized laboratories according to the protocol plan.
Typical Evaluation Laboratories
- Mechanical Evaluation Lab: Assesses device functionality, structural integrity, and wear.
- Analytical Chemistry Lab: Measures chemical composition, material breakdown, or extractables/leachables.
- Microbiology Lab: Validates sterility, bioburden, or preservative effectiveness.
- Packaging Lab: Verifies packaging performance after stress exposure.
- Photostability Lab (if applicable): Analyzes visual, material, or functional changes attributable to light exposure.
All test data are entered into a laboratory data management system for traceability, analysis by study scientists, and audit readiness.
Scientific Data Review
Stability scientists conduct rigorous data analysis from the test results:
- Prepare trend charts for the test results.
- Assess trends for out‑of-trend (OOT) or out‑of-specification (OOS) results.
- Compare data against acceptance criteria.
- Flag any unexpected findings and perform root‑cause investigations when necessary.
For each pull interval, generate an interim stability report . These summarize findings, identify trends, and provide early indicators of stability compliance or concern. Archive reports in your organization’s document control system for traceability and regulatory submission support.
6. Study Completion And Disposal
At the end of the planned study duration:
- A final stability report consolidates data from all pull points (Month 0 through the final time point).
- Stability scientists review accumulated results, address any OOT/OOS outcomes, and determine whether the study passes or fails relative to predefined acceptance criteria.
- Stability study results of the product are used for regulatory submissions pertaining to shelf life and life cycle changes.
- The study’s conclusion and data are documented, and the study is formally closed in LIMS.
Once official closure is complete:
- Remaining samples are removed from chambers and safely discarded following environmental and quality management policies.
Conclusion
A medical device stability study is a scientifically structured process that confirms a device’s quality over time and under environmental stress. When appropriately designed and executed, stability studies — including real‑time, accelerated excursion cycling, refrigerated/frozen, and photostability assessments per ICH Q1/Q1B — provide robust evidence supporting product shelf life claims, labeling, and regulatory compliance.
By adhering to internationally recognized guidance and leveraging digital systems such as LIMS and stability data management tools, manufacturers can produce high-confidence stability data that supports safe, effective, and reliable device performance across global markets.
About The Author:
 Venkatesan (Venkat) Muthukrishnan is a principal stability engineer at Johnson & Johnson MedTech, with over 20 years of experience in medical device R&D project management. He holds a Bachelor of Engineering in mechanical engineering, an Executive MBA, and professional certifications as a PMP and ASQ CSSBB. Venkat specializes in systems engineering, product development, and cross-functional project leadership, guiding programs from early concepts through launch while optimizing processes for efficiency, quality, cost, and regulatory compliance. He can be reached on LinkedIn.
Venkatesan (Venkat) Muthukrishnan is a principal stability engineer at Johnson & Johnson MedTech, with over 20 years of experience in medical device R&D project management. He holds a Bachelor of Engineering in mechanical engineering, an Executive MBA, and professional certifications as a PMP and ASQ CSSBB. Venkat specializes in systems engineering, product development, and cross-functional project leadership, guiding programs from early concepts through launch while optimizing processes for efficiency, quality, cost, and regulatory compliance. He can be reached on LinkedIn.
