Considerations For Rapid Prototyping In Medical Device Development
By Jim Kasic, Boulder iQ

It’s safe to say that rapid prototyping has taken the medical device industry by storm. True to its name, it is indeed quickly changing medical device development. Swift adoption and widespread use are allowing device developers to do things they’ve never done before and do them at a pace unheard of just a few years ago.
Yet the backdrop for rapid prototyping is not all sunshine and rainbows. As with most products and services, there are pros and cons. Device developers who understand these will be best positioned to benefit from the rapid prototyping process.
3D Printing
Rapid prototyping is the process of creating physical models or components of a device directly from digital designs. Since it generally uses 3D computer-aided design (CAD) input, it’s often simply referred to as 3D printing.
This process allows device manufacturers to create looks-like, feels-like, and works-like prototypes of complex devices and then develop iterations of those devices in a matter of hours. Plus, it all happens much less expensively than when using traditional methods of machining or injection molding.
So, what’s the problem? Why are there any “cons” at all? The issues derive primarily from two scenarios: when developers try to move directly into production with a 3D piece and when key stakeholders (such as investors) assume a 3D model is production-worthy, or closer to production than it really is.
Moving Into Production With A 3D Piece
The lure of accelerating the time to market and avoiding up-front costs of injection molding can tempt a device developer into trying to move directly into production with a 3D model. Consideration of some key facts may dull that lure.
- Biocompatibility retesting. Biocompatibility testing evaluates both the manufacturing process and the material. So, even if you’ve conducted full biocompatibility testing on a 3D model, you’ll likely need to repeat the testing later on when you move to injection molding. And any time you change the manufacturing process or change materials – common when moving from 3D to injection molding – you’ll need to redo at least some of the biocompatibility testing.
- Electrical and fire safety standards. For electronic devices that require enclosures, the enclosure material must meet designated electrical and fire safety standards. Not all 3D printing materials can meet those requirements. Because the required testing is time-consuming and expensive, manufacturers generally find it best to conduct the testing with the final device configuration.
- Sterilization requirements. Devices that need to be sterile must be able to endure a sterilization process. That can be a challenge for a plastic 3D-printed device, as sterilization may degrade or otherwise affect the part.
Sterile devices also must go through a shelf-life study and sterilization validation. Depending on the device and intended use, these can require hundreds of units. Plus, the 3D resin may not be compatible with the sterilization method or temperatures. As a result, the processes can be expensive and laborious with 3D-printed devices.
- Production cost. Rapid prototyping is fast and relatively inexpensive for 10, 20, or even 100 units. But for most parts or devices, once you move into volumes of a few hundred or more, it becomes uneconomical. On the other hand, with injection molding, the initial investment for the molds can be high. Once made, though, molding becomes significantly more cost-effective.
Let’s walk through a real-world example, looking at the cost to produce a single-use sterile articulating cardiac catheter device. You can see that even when adding in the cost of tooling, the cost per unit is still lower with injection molding than it is with 3D printing – and decreases dramatically at higher volumes.
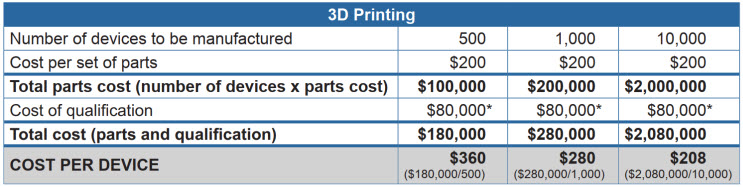
*Biocompatibility tests, sterilization validation, packaging/ship testing
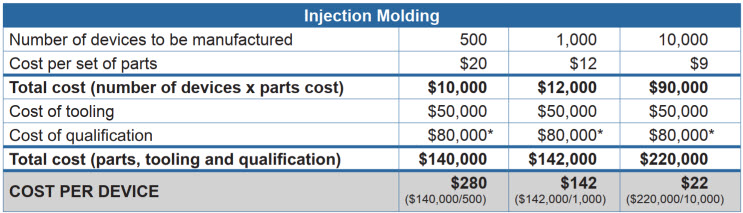
*Biocompatibility tests, sterilization validation, packaging/ship testing
Investor Understanding
The other issue involves very real concerns about investor confidence and expectations. Medical device developers are understandably anxious to satisfy investors and potential investors and prove that their devices can quickly become revenue-generating. But if investors mistakenly assume a 3D-produced model is production-worthy, or close to it, major funding and management consequences can arise.
For example, consider a startup making excellent progress on its device concept with the seed funding it received. When it comes time for an investor update, a skilled engineer works with a CAD program for a day and produces a file she sends to a 3D printer. Out comes a sleek, polished model that impresses investors in the update meeting. In response, eager to gain a competitive edge, the investors “highly recommend” that the company move quickly into production.
The problem is that a 3D model, while looking like a finished device, is not ready for production. The differences between a prototype and a device ready for market introduction are significant. Just because 3D printers can print almost anything doesn’t mean the results will be suitable for manufacturing. Even beyond all the factors mentioned above, keep in mind that 3D printers may print shapes that can’t be reproduced by any other means. Or they may print with parts that have undercuts that could never be removed from a production injection mold.
The investor audience must understand the context for any 3D model. If they don’t, the expectations may be far from reality, causing disappointment, frustration, and, sometimes, the end of the investor relationship.
Tips To Incorporate Rapid Prototyping Into Producible Design
Rapid prototyping has its pitfalls. But it also has a solid role in medical device development, with major benefits. Here are suggestions on how and when to incorporate 3D printing into the development process.
- Learn where 3D printing fits in your specific development process. Finding ways to speed development and get your device to market as quickly as possible are worthy goals, and there are ways to do so that include 3D printing. To start with, just creating an impressive concept model with 3D printing is a real milestone in device development. A 3D-printed model also can be a superb tool for trying out different aesthetic approaches.
Another use, producing fixtures and jigs, can be fast, relatively inexpensive, and a boon to the device manufacturing process. In addition, 3D models can help in human factors studies and preliminary market studies, such as by quickly creating several variations of a device for usability engineering sessions and focus groups.
The key is to plan ahead and consider where rapid prototyping works for your specific device in your development process. Then work that into the plan, allowing appropriate time for development and review of models.
- Plan to iterate. Rapid prototyping enables cost-effective, fast iterative testing and refinement prior to full-scale production. Using 3D printing early in the development process can significantly accelerate the process, reduce costs, and improve a device’s quality and effectiveness.
- Set expectations. If you are planning to use 3D printing anywhere in your development process, establish ground rules and expectations for how and when it will be used. Be sure senior management understands 3D’s role.
- Communicate. Once you have a realistic development schedule, communicate it broadly, and explain it clearly to all audiences. If one of those audiences is an investor audience, be sure you can explain, clearly and concisely, that the 3D-printed models they will admire will be only for visualization purposes, proof-of-concept, and early-stage testing,
Conclusion
Rapid prototyping has a well-earned space in your business. It allows device manufacturers to break new ground and do things that were previously impossible, from producing scale models to conducting market research that is more accurate than ever before. But before you plan to fly into rapid prototyping, take some time to understand how, when, and where to incorporate it into your development process. It can make the difference between market success and failure for your device.
About The Author:
 Jim Kasic is the founder and chairman of Boulder iQ. With more than 30 years of experience in the Class I, II, and III medical device industry, he holds more than 40 U.S. and international patents. His career includes experience with companies ranging from large multinational corporations to startups with a national and international scope. Kasic has served as president and CEO of Sophono, Inc., a multinational manufacturer and distributor of implantable hearing devices, which was acquired by Medtronic. He also was the president of OrthoWin, acquired by Zimmer-BioMed. He received a B.S. in physics and an M.S. in chemical/biological engineering from the University of Colorado and an MBA from the University of Phoenix. He can be reached at jim.kasic@boulderiq.com or on LinkedIn.
Jim Kasic is the founder and chairman of Boulder iQ. With more than 30 years of experience in the Class I, II, and III medical device industry, he holds more than 40 U.S. and international patents. His career includes experience with companies ranging from large multinational corporations to startups with a national and international scope. Kasic has served as president and CEO of Sophono, Inc., a multinational manufacturer and distributor of implantable hearing devices, which was acquired by Medtronic. He also was the president of OrthoWin, acquired by Zimmer-BioMed. He received a B.S. in physics and an M.S. in chemical/biological engineering from the University of Colorado and an MBA from the University of Phoenix. He can be reached at jim.kasic@boulderiq.com or on LinkedIn.
