Developing A Surgical Robotics System That Doctors Really Want — And Hospitals Can Afford
By Jim Pomager, Executive Editor

It’s hard to believe that 15 years have passed since the FDA approved the first robotic surgery platform for general laparoscopic surgery. Soon after that 2000 approval, Intuitive Surgical launched its da Vinci Surgical System, and the rest, as they say, is history. In the intervening years, the technology platform has completely transformed the field of minimally invasive surgery.
The benefits of surgical robots, among which da Vinci has become the unofficial standard, are many. Greatly enhancing the precision and dexterity of physicians during laparoscopic surgery, the technology has led to great reductions in procedure-related pain, discomfort, blood loss, infection, and scarring. As a result, patients are able to recover more quickly from procedures and resume normal activities. In addition, robotic surgery has enabled hospitals to lower pre- and post-operative care costs, as well as patient length of stay.
As a result, demand for and adoption of robotic surgery equipment predictably surged. According to iData Research, the combined market for surgical navigation systems and surgical robotic systems amounted to over $1.6 billion in 2013. The vast majority of that revenue was generated by the robotic assisted surgery segment, of which almost 90 percent was held by Intuitive. And iData expects the market for robotic assisted surgery equipment to reach $3 billion by 2020, experiencing double-digit growth over the next five years.
On the other hand, robotic surgery has also become something of a lightning rod for controversy in recent years. Intuitive has faced numerous liability lawsuits (and the resultant negative publicity) from patients alleging complications from surgeries performed with its products. Critics have repeatedly raised concerns about the efficacy of robotic surgery systems, especially in light of their cost — on average, a da Vinci system runs between $1.5 and $2.0 million, and that’s before factoring in the recurring expenses related to its disposable instruments and maintenance.
That high price tag is the main fetter restricting further growth of robotic-assisted laparoscopy, both in the United States and abroad. Here in the U.S., many small hospitals lack the procedure volume to justify the upfront investment in a robotic surgery system. And penetration outside the U.S., particularly in emerging markets, has been extremely limited. Of the 3,000+ da Vinci systems that have been installed to date, more than 2,000 of those are in the U.S. In order to reach a broader patient population, the cost of surgical robots will have to come down.
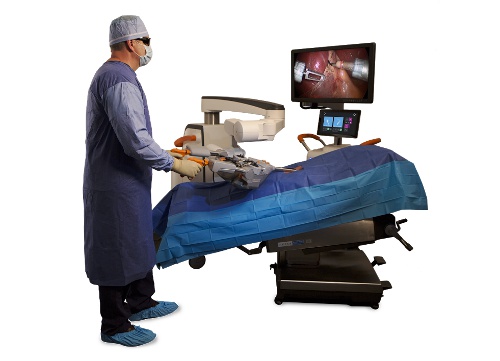
A new generation of robotic surgery platforms is being developed to address this need. In the laparoscopic surgery segment, perhaps the most noteworthy platform (and closest to commercialization) is the SurgiBot, created by TransEnterix. The North Carolina-based manufacturer has taken a most unconventional approach to its system design, at least compared to other players in the market. Instead of positioning the user at a control console located outside the sterile field, TransEnterix decided to return the surgeon to the patient’s bedside. The result is a robotic surgery system that is quite literally just what the doctor ordered — and that costs a fraction of existing platforms.
In a recent phone conversation, TransEnterix president and CEO Todd Pope explained to Med Device Online why he left his post at the top of a multibillion-dollar Johnson & Johnson company for a relatively unknown startup. He also described the rigorous process TransEnterix used to involve stakeholders from across the healthcare system in SurgiBot’s development, shared some of the lessons he learned along the way, and projects what he thinks the future will hold for the SurgiBot platform specifically, and for the robotic surgery in general.
Med Device Online (MDO): When and why did you join TransEnterix?
Todd Pope: I was the global president of Cordis, a division of Johnson & Johnson that will soon become part of Cardinal Health, prior to joining here in 2007. I always thought that starting something from scratch would be daunting, but also a great challenge and very rewarding.
So I actually came from running a very large, global company to TransEnterix, which was in its infancy at the time. I've been here from the beginning, and it's been a great ride.
MDO: What factors led the company to the development of its new SurgiBot platform?
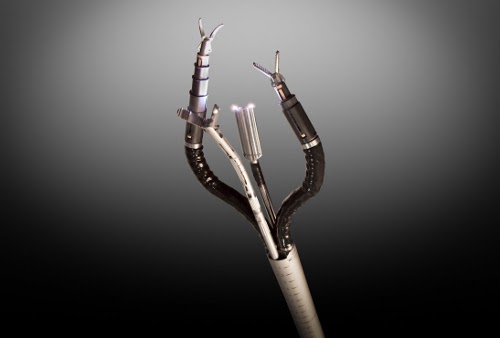
Pope: Well, we started off with the Spider, a single-port surgery platform that had four working channels going down one port. The feedback we got from physicians was universally positive. It really allowed them to do single-port surgery very similar to the way they did laparoscopic surgery, and that was the key.
To further enable the platform, surgeons told us that a couple features would be additive. They asked if it could be given greater strength, since it was a manual system at the time. They also wanted greater precision and advanced 3D vision. Lastly, with robotics, the surgeons hoped that we would allow them to operate in an ergonomically comfortable position. Laparoscopic surgery puts the surgeon in very difficult positions, they're standing beside the patient and reaching across them and operating in all four quadrants of the abdomen. The operating room is a very physically stressful environment for them.
Those were the categories that physicians came back to us and said, “If you can take your manual platform, the Spider, leave the patient interface the same, but give us some of these benefits on the back-end — by roboticizing it — that would be a fantastic addition to our surgery tools.”
So that's what we did. We leveraged our experience with the Spider and began to power it up and provide features that the surgeons had been asking about.
MDO: What limitations of existing robotic surgery platforms does SurgiBot seek to overcome?
Pope: We did a lot of market research, speaking with general surgeons who had experience with robotic surgery. We asked them what they liked the most and what areas they'd like to see improved. Far and away, the number one piece of feedback we got was that they wanted to remain in the sterile field, beside their patient.
Every surgery they've performed, every surgery they've ever trained on, they've been scrubbed in beside the patient. It is a comfort factor they’re used to. Their greatest request was to have the advantages of robotic surgery bedside but be beside the patient within the sterile field. That certainly was the overwhelming reason that we began to develop the SurgiBot the way we did. It’s completely different than other robotics platforms that are currently available or under development.
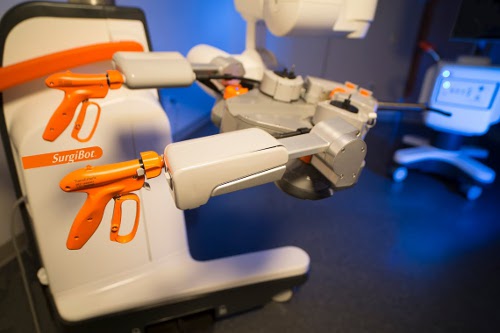
In addition, our product can easily move around the abdomen. Think about the abdomen as having four quadrants: upper left and right and lower left and right. Our system, very similar to a trocar, can move around the abdomen for multi-quadrant surgery. That was important, and platforms in the past had not been able to do that. Other platforms come straight from the top and operate on what is below. They aren't able to move around the abdomen freely, so we decided to incorporate that functionality just like our Spider has.
Surgeons also wanted a 3D camera that they could articulate, to get vision of anything in the operating field that they needed to see. They wanted a camera they could move left, right, up, and down, and we've developed that.
Lastly, many folks felt like robotics could be used in more facilities if it had a better price point. We really worked hard to get to a price point that is around $500,000 — 75 percent less than what other robotic surgery platforms cost today.
MDO: What applications are you initially targeting for the platform?
Todd Pope: We designed a system that we expect will have broad procedure applicability. It's really a mix of general surgery, bariatric surgery, colon and rectal, urology, and gynecology.
MDO: How close is SurgiBot to commercialization?
Pope: We plan to file our 510(k) in the middle of this year, and then we anticipate a six- to nine-month review process, so we hope to be approved and ready to start selling in the first half of 2016.
MDO: What do you expect the competitive landscape to look like upon SurgiBot’s launch?
Pope: We see no other surgical robotics platforms in development on the global scene that will keep the surgeon within the sterile field.
There are maybe one or two other platforms that are trying to replicate more of what Intuitive Surgical has done, but I don't think that they'll be to market in the near term.
MDO: What was the biggest challenge you faced in developing SurgiBot?
Pope: I think one of the greatest challenges for most medtech companies today is just the amount of time and money it takes to bring a product to market. It's quite an extensive process. The numbers of investors in medtech are down compared to years past. The regulatory hurdles are fairly steep, both here in the United States and around the world.
The main thing that young companies are facing today is the challenge of raising enough capital to adequately fund development to get the product on the market.
We've been fortunate to have very supportive investors from the venture capital community, and we now are public and are being supported well by public investors. That's one of the reasons we felt like we would have our best opportunity for success by going public — there's just a larger universe of public investors. That's worked out for us well.
MDO: What advice would you give to other young companies that are now facing this challenge?
Pope: No matter how conservative your estimates are, history shows that it takes most companies longer to get a product to market than they had originally anticipated, and it takes quite a bit more money. If you go back and look at the data, it typically plays out that way.
So you have to plan accordingly. If you're committed to your idea and your technology, and you think it's going to be of benefit to the medical community, you've got to adequately plan to have enough time and enough investor support to see your plans through.
MDO: Speaking of which, how long has TransEnterix been in development with SurgiBot?
Pope: We're in the third year of development of the product, but we also benefited from the four years we spent prior to that on Spider development and regulatory approval. We've been able to leverage that experience.
MDO: Some concerns have been raised over the past year or so regarding the risks, efficacy, and cost of robotic surgery. Have these issues had any impact on TransEnterix?
Pope: The robotic marketplace has been under scrutiny, but I actually think that's been a positive. The technology that's out there today continues to grow, it continues to be used in new applications, and that would not happen without good results and positive success. Even though the scrutiny is high, I think the early robotics market is really standing up quite well and credit has to be given to the pioneers.
The market certainly wants at least one other player. Often, when there's just one player in a market, they sometimes get unfair scrutiny, because there's no other option. I think us coming to market will actually be helpful to the robotics market as a whole, because we have a different approach, we have different features, and we have different price points.
We really look at our offering as expanding the market, not necessarily trying to compete head-to-head everywhere. We think we're going to be able to give a lot of people access to robotics that may not have it today or don't fully utilize it today.
MDO: Looking at the bigger picture, what industry trends have had the biggest impact on the way TransEnterix does business?
Pope: Over the past 10 or 20 years, medtech was primarily driven by interesting technology that was sold to surgeons, and then they drove the preference for that product. I think now, with many surgeons working for hospital systems, it's much more value-based decision making rather than individual preference.
The decision is more holistic across the hospital, and it includes quite a few different stakeholders — surgeons, administration, the executive branch of the hospital, patients, etc. There are many different stakeholders that weigh in now, before new technologies are brought to market, and I think that's ultimately a good thing.
MDO: Have you engaged these new stakeholders in the development of SurgiBot?
Pope: We certainly have, and one of the main ways we’ve responded over the last couple years is we've taken a step back and tried to drive efficiency in our development and come to market with a product that's at a different price point. That's the feedback we received from those stakeholders, that multi-million dollar robotics systems probably aren't ideal for every customer, not only for hospitals in the U.S. but in the international market as well.
They're excited about robotics internationally, but the technology currently has low penetration. If you get broad procedure applicability at a fair price, the technology is very enabling. You get the chance to reach more people. We did engage with folks early, and that's what drove our offering, both on features and price.
MDO: Have you filed for a CE Mark or other international regulatory approvals?
Pope: Right now, we're focusing on our 510(k). There are other areas around the world that accept the 510(k) approval.
The CE Mark is getting more and more difficult, compared to years past. There's not as big of a chasm between what needs to be done for a 510(k) and a CE mark, so we decided to start with the 510(k).
MDO: To what unique factors or approaches do you attribute TransEnterix’s success?
Pope: To be innovative, you certainly have to not only interface a lot with surgeons, but also with the different key stakeholders of the hospital. Then, you have to be able to develop a product fairly quickly to meet that feedback.
We have created an environment at TransEnterix where we can move quickly, and we've developed our own rapid prototyping shop. We have made a very large capital investment within our four walls, where we have lathes and mills and wire electrical discharge machining (EDM) equipment, laser welders, 3D printers — a tremendous capability that allows us to make almost every part for our product development in-house, so we don't have to go outside. This has been a very different approach that allows our development to move much more quickly than if we had to source the majority of our parts externally. The model has certainly paid dividends in SurgiBot’s development.
MDO: When you transitioning to full-scale manufacturing for SurgiBot, how much will you build in-house versus outsourcing?
Pope: We're doing it all in-house right now. Over the years, with volume, we'll look to outsource some of that, when appropriate, but we feel it's very important to keep both our development and manufacturing in-house.
MDO: Why is that?
Pope: When running a model that requires rapid innovation based on customer feedback, you can quickly fall behind if you don’t control the ability to make changes quickly. Our R&D department is connected to our rapid prototype shop, and when those two entities are working hand-in-hand, you can go faster and control the majority of the variables.
MDO: Looking ahead, what are the next steps for TransEnterix, and what do you think the future holds for robotic surgery?
Pope: We have some significant milestones coming up in the next 18 months that we're excited about. We're going to file our 510(k) for SurgiBot. We look forward to approval, our first commercial sale, and then really starting to gather post-market data on the product and its performance. We think it's going to be a compelling story, and we look forward to having the product out in the United States and in territories around the world.
Lastly, I would say that there are a lot of advantages robotics can deliver that haven't really reached the operating room yet, because of things we talked about earlier — there are high regulatory hurdles, and there are very large investments that need to be made. Sometimes that's difficult for young companies, but there are technologies that exist that we think will greatly improve surgery. We're very excited about being one of the companies to introduce some of that technology to the operating room in the coming years.
