Device Could Enable Pain-Free, At-Home Blood Collection — Without Needles
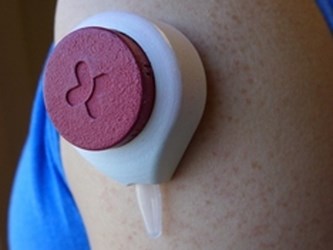
Startup company Tasso, Inc. is developing a device that uses suction to withdraw a small blood sample when held against the skin for two minutes. Users report that the process is virtually pain-free, and the developers say the device could provide safe and convenient blood draws in a home-care environment.
Hemolink is a microfluidic platform designed for self-collection of blood samples, and is approximately the size of a ping-pong ball. Instead of accessing blood from vessels as needles do, Hemolink uses a small amount of vacuum suction to draw blood from closer to the surface of the skin in the capillary bed. The device can draw 200 mL of blood into an attached tube, enough to test for cholesterol, infection, cancer cells, and blood sugar.
“At these scales, surface tension dominates over gravity, and that keeps the blood in the channel no matter how you hold the device,” said Ben Casavant, one of the founders of Tasso and developer of the device, in an article published by the University of Wisconsin-Madison News.
Casavant said that moving blood in open channels rather than closed ones simplifies manufacturing. Their device is disposable and needs as few as six injection-molded plastic parts.
In a press release last year, Tasso announced it had received $2.3 million in grant funding from the Defense Advanced Research Projects Agency (DARPA) and the National Institute of Health (NIH).
“We have compelling data, a motivated management team, and an unmet clinical need in a growing market,” said Ben Moga, Tasso’s president. “Expanding home care by enabling safe and convenient blood draws for clinical diagnosis and monitoring is exactly the kind of innovation that improves outcomes without raising healthcare costs.”
According to a Tasso press release earlier this month, the company has secured an additional $3 million in grant funding from DARPA. Researchers plan to use this money to collaborate with GenTegra, a company that manufacturers blood preservatives.
Experts claim that if the collected blood samples could be stabilized in a 140-degree environment for one week and still remain useable, the device could feasibly be sent through the mail for analysis without requiring refrigeration.
Bruce Jamieson, CEO of GenTegra, said in the press release, “The combination of a simple blood collection device and stabilization of the specimen at ambient temperatures will open many possibilities for new innovative ‘in home’ and ‘point-of-care’ sample collection scenarios.”
Though there are many potential applications for the technology, the developers have not narrowed down a specific market yet and said they are keeping their options open. Casavant said in the University of Wisconsin-Madison News article that he and his associates plan to submit a premarket application to the FDA by the end of this year and market the device as early as 2016.
Image credit: David Tenenbaum, University of Wisconsin-Madison News
